Chemical properties of Aluminiumuction
The availability of aluminium in a pure state is very rate due to its chemical properties. Additionally, it is one of the most abundant compounds on the Earth due to its availability of it as a compound state with other materials. “Alum and aluminium oxide” are the two most frequent particles discovered as the chemical properties of this material. This tutorial helps understand the usefulness of aluminium in the different prospective and shares knowledge regards the chemical properties of aluminium.
Information about Aluminium
The electron number of the aluminium is 13, which refers to that; it has exactly 13 protons in the nucleus of an atom of aluminium. The most useful characteristic of aluminium is it can combine with over 270 materials found in nature. Due to the softness and durability of this material, it has enormous use cases. Aluminium is not a magnetic material and it is not resistant to corrosion. In the right state, aluminium can dissolve in the water. The aluminium comes as a member of the "Boron family" which has the electronic configuration of "" (Ponnusamy et al. 2020).
With the inclusion of the "group 13" material, aluminium can form an ionic of the "A13+ ions".
Images Coming soon
Figure 1: Information about Aluminium
One of the major characteristics of aluminium is, that it has a very less amount of density in the chemical reaction that is making this element soft and durable and possible to use in "multiple formations". The chemical and biological application of aluminium is constructing it as multi-perspective material on Earth. The benefits of using this material can help in manufacturing different items, such as cans, parts for aeroplanes and rockets, kitchen utensils, foils, and different other materials that have usefulness in the different industries. Different forms of aluminium have applications in manufacturing cars, watches, bicycles, railway engines, and other materials (Matmatch, 2022).
The usefulness of the material is due to its lightweight and it is possible to form the desired shapes easily with the aluminium.
Images Coming soon
Figure 2: "Properties and application of Aluminium"
Chemical Properties of Aluminium
The following illustrations are regarding the first chemical properties of Aluminium: The reaction of aluminium with air is depending on the oxide layer that is to protect the coating of the aluminium from wiped out due to the attack made by the air. If the "oxide layer" gets hurt, the vulnerability of the Aluminium metal can be witnessed which reacts with oxygen and benefits in the appearance of "amphoteric oxide".
Another reaction with the acids can make the formula of the hydrogen gas. The chemical properties of the aluminium are the reaction with another component. In this formation, mineral acids are reactive to make the solutions that may have liquid along with releasing H2. The reaction between HCL and Aluminium will also create releasing the hydrogen gas.
The third chemical property of the aluminium material is the formation of a new compound. Oxygen and hydrogen have different chemical properties that can make the items significant in the present circumstances (Mwema et al. 2018).
"Oxygen and Aluminium electro negativity" can construct it feasible for the Aluminium to compose bonds that are covalent and able to react with oxygen. Aluminium reacts to the items that are warm and can create a colourless solution of sodium.
Images Coming soon
Figure 3: Chemical properties of Aluminium
The followings are the unique features of the Aluminium that comes under the chemical properties due to the behaviour:
Aluminium is a material that consists of the "silver type" and includes a bluish colour.
The melting point of the Aluminium is 660°C.
Aluminium has a density of "2.708grams/cubic centimetre".
Application of Aluminium
Different applications of Aluminium are applicable in the different industries to create materials that are intended to be most durable. The availability of aluminium on the Earth is around eight per cent crust in the earth. Different types of metal can form different states of items that can make synthetic materials. Aluminium is one of the stable materials that is found for the construction of different materials. The utilisation of aluminium is not used for the construction of the different materials that are not sustainable as aluminium is not a stable material. Additionally, combinations with other materials such as magnesium, manganese, copper, and silicon are lightweight but create a stable formation (Smith et al. 2018). They are effective in the construction of different materials that are generally used in aeroplanes and these materials are used in distinct classes of transportation.
Isotopes
"Aluminium has six radioactive isotopes." The naturally occurring isotopes of aluminium refer to the aluminium-27. Isotopes are forming about the formation of a new element. The "mass number" refers to the presentation of the neutrons and protons in an atom's nucleus. Significantly, according to chemistry, the protons determine the characteristic, but the "number of the neutrons in the atom" can vary depending on the number. Different numbers are the reference of an isotope for a material.
Conclusion
This tutorial highlights the chemical properties of one of the most abundant material presents on the Earth, Aluminium. It has a less amount of density and its durability of it can make the development of the different significant items. Most of the time, the combination of the other material comes useful to create the useful application of Aluminium. Due to modern welfare, the application of aluminium is available in the construction of the different items.
Frequently Asked Question (FAQs)
Q1. What is the number of ions available in Aluminium?
The number of ions present in the Aluminium is 13, which seems that Aluminium has 13 electrons and 13 protons in each atom.
Q2. What are the properties of aluminium?
Aluminium has a low density, the conductivity of the material is highly thermal, and it is a non-toxic material.
Q3. What are the possible applications of Aluminium?
The construction of stable and lightweight material refers to the application of aluminium.
In different transportation use cases, aluminium is replacing steel for the property which is effectively stable and lightweight.
References
Journals
Ponnusamy, P., Rahman Rashid, R. A., Masood, S. H., Ruan, D., & Palanisamy, S. (2020).
Mechanical properties of SLM-printed aluminium alloys: a review. Materials, 13(19), 4301. Retrieved from: https://www.mdpi.com/1996-1944/13/19/4301/pdf
Smith, S. R., Rafati, R., Haddad, A. S., Cooper, A., & Hamidi, H. (2018). Application of aluminium oxide nanoparticles to enhance rheological and filtration properties of water based muds at HPHT conditions. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 537, 361-371. Retrieved from:https://aura.abdn.ac.uk
Websites
Chemistryexplained, (2022), Aluminium, Retrieved from: http://www.chemistryexplained.com/a> [Retrieved on 23rd June 2022]
Matmatch, (2022), Chemical properties and application of Aluminium, Retrieved from: https://matmatch.com [Retrieved on 23rd June 2022]
Twi, (2022), materials from Aluminium, Retrieved from: https://www.twi-global.com [Retrieved on 23rd June 2022]
Chemical properties of Metals
Introduction
At present, there are 118 elements that are known to us and from them, 92 elements occur naturally. The other metals within the total list are prepared with the help of artificial methods. However, elements are classified into several categories, that includes, metals, metalloids and non-metals based on their respective properties and are correlated with respect to their periodic table. There are several physical and chemical properties, that are noticed among metals.
What are metals?
Both metals and non-metals are found within our surroundings and are quite essential in their presence. Metals can be defined as the substances that have unique characteristic properties, such as sonority, solidness, conductivity, malleability, and ductility (Bedassa & Desalegne, 2020).
Some examples of metals include zinc, aluminium, iron, tin, titanium, copper and many more. Generally, it is noticed that metals are good conductors of thermal conductivity and conductivity to electricity. However, following the periodic table, the nature of the metals is acknowledged, as they are placed in a unique way depending upon their properties.
Images Coming soon
Figure 1: Origin of ores
Physical and Chemical properties of metals
Physical properties of metals include the state of matter that means at what state they are found in the room temperatures, malleability, and hardness. The density of metals is quite high and their valences remain from 1 to 3 in the outermost shells; they also seem to possess high melting and boiling points (Proshad et al. 2021).
Metals are quite a lustre in nature for example, gold, and copper. On the other hand, the chemical properties of metals include the formation of alloys with both metals and nonmetals. Metals burning in presence of oxygen within air lead to generating metal oxides. Highly reactive metals such as, sodium and potassium are stored in oil as they react at a faster rate in any kind of chemical reaction (Geeksforgeeks, 2022). However, metals also generate metal oxides and hydrogen gas at the time of reacting with water.
Images Coming soon
Figure 2: Properties of metals
There occurs production of salt and hydrogen when metals react with acidic elements. However, within the solution of metal salt, the metals tend to displace other metals that are less reactive in nature.
Images Coming soon
Figure 3: Properties of metals and non-metals
What are non-metals?
Non-metals are those natural elements that are not capable of generating electricity and heat. The non-metals have structures that are structurally brittle in nature and they cannot be rolled, pressed, module and extruded easily. The non-metals include, hydrogen, arsenic, phosphorous, nitrogen, oxygen, and selenium present within the periodic table.
Reaction with metals
- Reaction of metal with oxygen
In reacting with oxygen, the metals tend to produce metal oxides, as they tend to donate the lone pairs of electrons to the atom of oxygen while reacting. For example, However, it is noticed that oxides of metal are basic in nature and often at times display the amphoteric behaviour (Tutormate, 2022). Amphoteric behaviour results in displaying both the acidic and basic characteristics.
- Reaction of metal with water
In reacting with water, metals tend to generate metal hydroxides, however, it should be noted that some metals are non-reactive to water. Therefore, the rate of reactivity of water varies from one metal to another. For example,
- Reaction with dilute acid
While reacting with dilute acids, metals tend to pop sounds due to the production of hydrogen gas. However, metal salts are also produced while reacting with acids. For example, such reactions are, . The metals that are located in the position low to the hydrogen with the series of reactivity tend to react with dilute acids (Chem.libretexts, 2022). However, these metals are quite unable to displace hydrogen and are unable to form a bond with the anion of non-metal.
- Reaction of metals with other metal salts
It is noted that metals that have high reactivity tend to replace less reactive metals while conducting the reaction. Such example includes, less reactive metals, like, sulphides, and chlorides. For example, such reaction is,
Conclusion
In this tutorial, the focus has been given to identifying the chemical properties that are noticed within metals. However, the differentiation is made for both metals and non-metals, discussing their innate properties, such as metals showing high conductivity of electricity whereas; non-metals are unable to display such characteristics. On the other, metals tend to have solid structures, whereas, non-metals are brittle in nature. Certain chemical reactions are discussed that will help in a better understanding of the chemical properties possessed by metals.
Frequently Asked Questions (FAQs)
Q1. What are the examples of metals and non-metals?
In the periodic table, there lie many examples for metals as well as non-metals. Examples of metals include, potassium, sodium, iron, thorium, uranium, tungsten, caesium, aluminium, and cadmium zinc and many more. On the other hand, some examples of non-metals include several types of polymers and elastomers such as mica, polycarbonate, garnet, agate, and all kinds of rubbers. The elements such as, fluorine, helium, xenon, and iodine and the elements that are related to this are known as non-metals.
Q2. What are the physical properties noticed for metals present within the periodic table?
Several physical properties '' are there, that are exhibited by metals and with these properties, the metals can be easily distinguished from non-metals. Such properties include, high melting points, ductile nature, malleability, and high density. More to this, metals are good conductors of both heat and electricity.
Q3. What is defined as a melting point?
Melting point is defined as the temperature, when the metals start to melt and display a pure metal. For example, the melting point of aluminium is 660° C, brass is 930° C, and stainless steel is, 1375° to 1530° C and many more.
Reference
Journals
Bedassa, T., & Desalegne, M. (2020). Assessment of Selected Physico-Chemical Properties and Metals in Qeera Stream Water, Bakkee-Jamaa, Nekemte, Ethiopia. International Journal of New Chemistry, 7(1), 47-59. Retrieved from: http://www.ijnc.ir/&url=http://www.ijnc.ir/article_37717.html
Proshad, R., Zhang, D., Idris, A. M., Islam, M., Kormoker, T., Sarker, M. N. I., ... & Islam, M. (2021). Comprehensive evaluation of chemical properties and toxic metals in the surface water of Louhajang River, Bangladesh. Environmental science and pollution research, 28(35), 49191-49205. Retrieved from: https://link.springer.com/article/10.1007/s11356-021-14160-6
Websites
Chem.libretexts, (2022), Metals and non-metals , Retrieved from: https://tutormate.in/cbse-class-10-chemistry/chemical-properties-of-metals Retrieved from: https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Chemistry_-_The_Central_Science_ (Brown_et_al.)/07%3A_Periodic_Properties_of_the_Elements/7.06%3A_Metals_Nonmetals_and_Metalloids [Retrieved on, June 2022]
Geeksforgeeks, (2022), Chemical properties of metals and non-metals, Retrieved from: https://www.geeksforgeeks.org/chemical-properties-of-metals-and-non-metals/ [Retrieved on, June 2022]
Thoughtco, (2022), Chemical properties of matter, Retrieved from: https://www.thoughtco.com/chemical-properties-of-matter-608337 [Retrieved on, June 2022]
Tutormate, (2022), Chemical properties of metals and non-metals, Retrieved from: https://tutormate.in/cbse-class-10-chemistry/chemical-properties-of-metals/ [Retrieved on, June 2022]
Chemical properties of Carbon Compounds
Introduction
In chemistry, carbon is considered a very surprising and different element. The symbol of this element is C and the atomic number is 6. This element is known as a non-metallic tetravalent element. This element has a valence of 4 that makes the element enable to enter into covalent bonding with the other properties, based on these attributes of carbon, the present tutorial will discuss the properties of carbon and its chemical properties of it as well.
Carbon Properties: Definition
Images Coming soon
Figure 1: Carbon atom
In chemistry, it has been believed that the element carbon has an unusual capacity that enables the elements to create a bond with the atoms of other carbon. This reaction helps the atoms to generate more complex molecules. These characteristics of the molecules of carbon properties are termed Catenation (Kokorina et al. 2020).The complex molecules have been formed on the basis of the size of the atom that is too small to form such molecules. In the outer shell of every atom, there are four valence electrons have been found. These electrons are considered responsible for generating the formation of chemical bonds with other atoms and molecules.
Combustion of Carbon Compounds
In accordance with the diction of chemistry, the process of burning is usually referred to as the process of combustion. When a carbon compound is burnt in the air, it produces, water, heat, light and carbon dioxide. In some cases, vapours have been used after being burnt in the air as the reaction can be represented as Hydrocarbon + Oxygen = Heat energy (Aftanaziv et al. 2022).
For an instance of such a reaction, Alkanes can be taken as the burning of such compounds generates lots of heat and for this particular reason; this is considered a good fuel. The equation of such reactions can be
Oxidation of Carbon Compounds
In the particular reaction where it has been observed that a hydrogen atom is lost, on the contrary, an addition of an oxygen atom happens; the reaction will be termed Oxidation. However, it can be observed that not every reaction adds oxygen by losing a hydrogen atom (Harvard, 2022).
The reaction, where such a phenomenon has been observed is represented as This specific reaction suggests that the chemical reaction has been conducted between copper and oxygen which generates a new element called copper oxide.
Addition of Carbon Compounds
In the Addition of Carbon Compounds, the reaction takes place between an atom of unsaturated hydrocarbon and hydrogen that can generate a singular product (Jegatheesan & Rajasekaran, 2021). This type of reaction is generally taking place in the presence of the catalysts of palladium or nickel.
Images Coming soon
Figure 2: Effect of catalyst
Catalysts are referred to as the particular substance that is responsible for the reaction to occur or proceed at a rate that is not similar to the rate for which the reaction itself can be affected (Sciencedirect, 2022). Based on the above figure, it can be stated that the presence of a metal catalyst, like rhodium, palladium, platinum and many more, the reaction boosts the rate of the reaction in a drastic manner.
Substitution of Carbon Compounds
This specific type of reaction within the carbon compounded is found in such kinds of reactions where the involvement of a less reactive element is observed with the more reactive element. This reaction is mostly observed in the reactions of hydrocarbons that are hydrocarbons. These reactions are usually considered single displacement reactions (Sciencedirect, 2022). This reaction can be represented as a chemical reaction. The chemical representation of such kind of reaction can be represented as This reaction represents the chemical reaction that is observed between methane and chlorine. The electrons of methane are considered less reactive in comparison to the more reactive element chlorine. Based on these aspects, it can be stated that the atoms of chlorine have the ability to create a displacement of hydrogen atoms of the atomic formulation of the saturated hydrocarbons. In this particular process, the element that will be generated in a frequent manner is Higher homologue.
Conclusion
The present tutorial has included an explanation of the carbon compounds and the several chemical properties that are generally applied for different chemical reactions. The tutorial has further included an explanation regarding the process named Combustion of Carbon Compounds. The other chemical compounds that have been explained in accordance with the reactions are found while addition of oxygen, is commonly known as oxidisation and the Substitution of Carbon Compounds. The explanation of Addition of Carbon Compounds has included the reactions that are mostly seen between unsaturated hydrocarbon and hydrogen where a single product can be generated.
FAQs
Q1. Which of the following hydrocarbons undergo an addition reaction with C2H6, C3H8, C3H6, C2H2 and CH4?
According to chemistry, the addition reaction takes place in unsaturated hydrocarbons. Therefore, the reaction can take place with the elements like and because both the compounds have a double bond between the bond of carbon compounds.
Q2. Why are the compounds of carbon applied as fuels?
The main reason for applying the carbon compounds as fuels are that the carbon can produce a clean flame and after the reaction, dioxide and water are produced. Moreover, no smoke is evolved during the combustion but the heat and light are evolved.
Q3. What is the type of Covalent Bond?
The single covalent bond refers to the molecular bond where a single pair of electrons is shared between two atoms. The reaction between two pairs of electrons and two atoms is referred to as a Double Covalent Bond. At times when three pairs of electrons are shredded between two molecules of an atom, the reaction is called a Triple Covalent Bond.
References
Journals
Aftanaziv, I., Malovanyy, M., Shevchuk, L., Strogan, O., & Strutynska, L. (2022). Economic and Environmental Benefits of Using Cavitation Treated Fuel in Vehicles of Internal Combustion Engines. COMMUNICATIONS, 24(3), B158-B169. Retrieved from: https://scholar.archive.org
Jegatheesan, A., & Rajasekaran, E. (2021, November). Characterization of Organic Compound Doped Inorganic Ammonium Phosphate: Crystal Formation and Opto-Electrical Properties. In Journal of Physics: Conference Series (Vol. 2070, No. 1, p. 012004). IOP Publishing. Retrieved from: https://iopscience.iop.org
Kokorina, A. A., Ermakov, A. V., Abramova, A. M., Goryacheva, I. Y., & Sukhorukov, G. B. (2020). Carbon nanoparticles and materials on their basis. Colloids and Interfaces, 4(4), 42. Retrieved from: https://www.mdpi.com/2504-5377/4/4/42/pdf
Websites
Harvard, (2020). Oxidation of carbon compounds by silica. Retrieved from: https://ui.adsabs.harvard.edu/abs/2013EP&S...65..811I/abstract [Retrieved on 27th June 2022]
Sciencedirect, (2022). Carbon Atom. Retrieved from: https://www.sciencedirect.com/topics/chemistry/carbon-atom [Retrieved on 27th June 2022]
Sciencedirect, (2022). Effect of catalyst on energy diagram profile. Retrieved from: https://www.researchgate.net [Retrieved on 27th June 2022]
Bond Energy
Introduction
The amount of energy produced when one mole of bonds is created in isolated gaseous atoms to form a gaseous compound is known as bond formation energy or bond energy. The amount of energy necessary to break the link between two gaseous compounds and generate isolated gaseous atoms is known as bond dissociation energy. These two values are usually the same for a diatomic molecule, hence the phrase "bond energy" is employed. The phrase ‘average bond energy’ is used to describe the bond energy of a polyatomic molecule.
Bond Energy Corresponding to a Chemical Bond
The average bond energy involved with breaking the individual bonds of a molecule is measured by measuring the heat necessary to split one mole of molecules into their constituent atoms. The bond between the two atoms is said to be 'stronger' when the bond energy is higher, and the distance between them (bond length) is lower.
The HO-H bond in a water molecule, for example, needs 494 kJ/mol to break and produce the hydroxide ion (OH–). An extra 425 kJ/mol is required to break the O-H bond in the hydroxide ion.
As a result, the average of the two values, or 459 kJ/mol, is given as the bond energy of covalent O-H bonds in water. The energy values necessary to break successive O-H bonds in the water molecule are known as 'bond dissociation energies,' and they differ from the bond energy. The bond energy is the sum of a molecule's bond dissociation energies.
The nature of the other bonds in the molecule influences the exact parameters of a certain form of bond; for example, the energy and length of the C–H bond change depending on which other atoms are connected to the carbon atom. Similarly, the length of the C-H bond can vary by as much as 4-5 % between molecules.
As a result, the values reported in bond energy and bond length tables are often averages of a range of compounds containing a specific atom pair.
| Bond | Bond length (angstrom) | Bond energy (kJ/mol) |
|---|---|---|
| C-C | 1.54 | 348 |
| C=C | 1.34 | 614 |
| C≡C | 1.20 | 839 |
Calculating Bond Energy
- For diatomic molecule (HCl)
HCl is formed as result of association between hydrogen and chlorine gas as shown below:
The bond energy corresponding to each bond has been mentioned below in the table:
| Bond | Bond energy (kJ/mol) |
|---|---|
| H-H | 437 |
| Cl-Cl | 244 |
| H-Cl | 433 |
Energy Change = (437 + 244) – 2 × 433 kJ/mol = (681 – 866) kJ/mol = - 185 kJ/mol
- For polyatomic molecules
Let us calculate the bond energy for O-H bond in a water molecule. The reaction can be represented as
The bond energy of every O-H bond in water molecules could be considered as the average bond energies of every individual O-H bond. It could be calculated in the following manner:
Where, indicates the energy required to break one O-H bond in and indicates the energy required to break one O-H bond in OH.
Factors Affecting Bond Energy
As the atom's size increases, the bond length increases and the bond energy decreases, lowering bond strength.
The bond energy of a bond between two identical atoms increases as the bond multiplicity increases.
As the number of lone pairs of electrons on bonded atoms increases, the repulsion between them increases, and the bond energy decreases.
As the bond energy increases, the s orbital contribution on the hybrid orbital increases. As a result, bond energy drops in the sequence listed below:
The higher the electronegativity difference, the higher the bond polarity and hence the bond strength, or bond energy. Thus, halides follow the order:
Conclusion
Bond Energy is a measurement of the bond strength required to disassemble one mole of a compound into its component atoms. The bond enthalpy, or average bond enthalpy, is another name for it. The stability of a chemical bond is directly proportional to its bond energy.
FAQs
Q1. Define Bond Energy.
Ans: The amount of energy produced when one mole of bonds are created in isolated gaseous atoms to form a gaseous compound is known as bond formation energy or bond energy.
Q2. What is the difference between bond energy and bond dissociation energy?
Ans: Bond Dissociation Energy would indicate the amount of energy required to break down a particular bond in hemolysis. While Bond Energy refers to the average amount of energy necessary to disassemble all the bonds which exist between same two types of atom in a compound. For a diatomic molecule, the bond energy is equal to bond dissociation energy.
Q3. What would be the expression of Bond Energy for a C-H bond in ?
Ans: The expression for Bond Energy in would be given as:
Where BE1 indicates the bond energy required to break one C-H bond in indicates the bond energy required to break one C-H bond in indicates the bond energy required to break one C-H bond in indicates the bond energy required to break one C-H bond in CH.
Q4. What are the factors that could affect Bond Energy?
Ans: The factors effecting bond energy are:
(i) Atomic Radius
(ii) Electronegativity
(iii) Number of Lone Pair on bonded atoms
Q5. What would be the effect of polarity on bond energy?
Ans: A more polar bond would have greater separation of charge (dipole moment) between the two atoms due to greater difference in electronegativity. Thus, the bond will have greater ionic tendencies compared to the covalent character. As the polarity increases, the bond energy would increase.
Bond Energy
Introduction
The amount of energy produced when one mole of bonds is created in isolated gaseous atoms to form a gaseous compound is known as bond formation energy or bond energy. The amount of energy necessary to break the link between two gaseous compounds and generate isolated gaseous atoms is known as bond dissociation energy. These two values are usually the same for a diatomic molecule, hence the phrase "bond energy" is employed. The phrase ‘average bond energy’ is used to describe the bond energy of a polyatomic molecule.
Bond Energy Corresponding to a Chemical Bond
The average bond energy involved with breaking the individual bonds of a molecule is measured by measuring the heat necessary to split one mole of molecules into their constituent atoms. The bond between the two atoms is said to be 'stronger' when the bond energy is higher, and the distance between them (bond length) is lower.
The HO-H bond in a water molecule, for example, needs 494 kJ/mol to break and produce the hydroxide ion (OH–). An extra 425 kJ/mol is required to break the O-H bond in the hydroxide ion.
As a result, the average of the two values, or 459 kJ/mol, is given as the bond energy of covalent O-H bonds in water. The energy values necessary to break successive O-H bonds in the water molecule are known as 'bond dissociation energies,' and they differ from the bond energy. The bond energy is the sum of a molecule's bond dissociation energies.
The nature of the other bonds in the molecule influences the exact parameters of a certain form of bond; for example, the energy and length of the C–H bond change depending on which other atoms are connected to the carbon atom. Similarly, the length of the C-H bond can vary by as much as 4-5 % between molecules.
As a result, the values reported in bond energy and bond length tables are often averages of a range of compounds containing a specific atom pair.
| Bond | Bond length (angstrom) | Bond energy (kJ/mol) |
|---|---|---|
| C-C | 1.54 | 348 |
| C=C | 1.34 | 614 |
| C≡C | 1.20 | 839 |
Calculating Bond Energy
- For diatomic molecule (HCl)
HCl is formed as result of association between hydrogen and chlorine gas as shown below:
The bond energy corresponding to each bond has been mentioned below in the table:
| Bond | Bond energy (kJ/mol) |
|---|---|
| H-H | 437 |
| Cl-Cl | 244 |
| H-Cl | 433 |
Energy Change = (437 + 244) – 2 × 433 kJ/mol = (681 – 866) kJ/mol = - 185 kJ/mol
- For polyatomic molecules
Let us calculate the bond energy for O-H bond in a water molecule. The reaction can be represented as
The bond energy of every O-H bond in water molecules could be considered as the average bond energies of every individual O-H bond. It could be calculated in the following manner:
Where, indicates the energy required to break one O-H bond in and indicates the energy required to break one O-H bond in OH.
Factors Affecting Bond Energy
As the atom's size increases, the bond length increases and the bond energy decreases, lowering bond strength.
The bond energy of a bond between two identical atoms increases as the bond multiplicity increases.
As the number of lone pairs of electrons on bonded atoms increases, the repulsion between them increases, and the bond energy decreases.
As the bond energy increases, the s orbital contribution on the hybrid orbital increases. As a result, bond energy drops in the sequence listed below:
The higher the electronegativity difference, the higher the bond polarity and hence the bond strength, or bond energy. Thus, halides follow the order:
Conclusion
Bond Energy is a measurement of the bond strength required to disassemble one mole of a compound into its component atoms. The bond enthalpy, or average bond enthalpy, is another name for it. The stability of a chemical bond is directly proportional to its bond energy.
FAQs
Q1. Define Bond Energy.
Ans: The amount of energy produced when one mole of bonds are created in isolated gaseous atoms to form a gaseous compound is known as bond formation energy or bond energy.
Q2. What is the difference between bond energy and bond dissociation energy?
Ans: Bond Dissociation Energy would indicate the amount of energy required to break down a particular bond in hemolysis. While Bond Energy refers to the average amount of energy necessary to disassemble all the bonds which exist between same two types of atom in a compound. For a diatomic molecule, the bond energy is equal to bond dissociation energy.
Q3. What would be the expression of Bond Energy for a C-H bond in ?
Ans: The expression for Bond Energy in would be given as:
Where BE1 indicates the bond energy required to break one C-H bond in indicates the bond energy required to break one C-H bond in indicates the bond energy required to break one C-H bond in indicates the bond energy required to break one C-H bond in CH.
Q4. What are the factors that could affect Bond Energy?
Ans: The factors effecting bond energy are:
(i) Atomic Radius
(ii) Electronegativity
(iii) Number of Lone Pair on bonded atoms
Q5. What would be the effect of polarity on bond energy?
Ans: A more polar bond would have greater separation of charge (dipole moment) between the two atoms due to greater difference in electronegativity. Thus, the bond will have greater ionic tendencies compared to the covalent character. As the polarity increases, the bond energy would increase.
Difference Between Cis and Trans
The terms "cis" and "trans" are used in various fields of science and social studies to describe different aspects of objects, molecules, and people. In chemistry, these terms are used to describe the orientation of atoms or groups of atoms in a molecule, while in social studies, they are used to describe the gender identity of individuals. In this essay, we will discuss the difference between cis and trans in both chemistry and social studies.
What is Cis?
The prefix “cis” is derived from Latin. It means “on the same side”. In the cis isomer, the substituent groups are placed on one side of a double bond plane or a non-aromatic cycle.
Cis and trans isomers differ in their physical properties, due to the inequality in the overall dipole moment and the molecules’ shape.
The relative boiling point is being determined by the polarity. It causes increased intermolecular forces, which results in an increase of the boiling point. The cis isomers, which are more polar than the trans isomers, have a higher boiling point. The difference can be small, as it is in the alkenes with straight chain. Larger difference is observed in substances with polar bonds. Example for such substance is the 1,2-dichloroethene. The boiling point of its cis isomer is 60.3 °C, and of its trans isomer – with 12.8 °C lower. The reason for the difference is that in the cis isomer the two C-Cl polar bonds’ dipole moments couple and produce an overall molecular dipole. As a result, there occurs intermolecular dipole–dipole forces, which raise the boiling point.
The symmetry allows for better packing of the solid substances. As a result of the different symmetry of the molecules, the cis and trans isomers differ in their melting points. The cis isomers, which are less symmetrical, have a lower melting point, compared to the trans isomers.
What is Trans?
The prefix “trans” is derived from Latin. It means “on opposing sides”. In the trans isomer, the substituent groups are placed on different sides of a double bond plane or a non-aromatic cycle.
The boiling point of the trans isomers is lower than in the cis isomers. The difference is more significant in substances with polar bonds. In the trans isomer of 1,2-dichloroethene, the two C−Cl bond moments cancel each other and the molecule has a net zero dipole. As a result, there are no intermolecular dipole–dipole forces, which decrease the boiling point.
The symmetry of the molecules is the key in the determination of the melting point, due to the better packing of the solid substances. Examples of this are the oleic acid (cis isomer) and elaidic acid (trans isomer). The cis isomer’s melting point is 13.4 °C, the trans isomer melts at 43 °C. The reason for this is that the trans isomer is straighter, packs better, and hence – having a much higher melting point.
The trans isomers have lower densities than their cis counterparts. In acyclic systems, trans isomers are more stable than cis isomers. In general, cis isomers have higher solubility in inert solvents.
The prefix “trans” is derived from Latin. It means “on opposing sides”. In the trans isomer, the substituent groups are placed on different sides of a double bond plane or a non-aromatic cycle.
The boiling point of the trans isomers is lower than in the cis isomers. The difference is more significant in substances with polar bonds. In the trans isomer of 1,2-dichloroethene, the two C−Cl bond moments cancel each other and the molecule has a net zero dipole. As a result, there are no intermolecular dipole–dipole forces, which decrease the boiling point.
The symmetry of the molecules is the key in the determination of the melting point, due to the better packing of the solid substances. Examples of this are the oleic acid (cis isomer) and elaidic acid (trans isomer). The cis isomer’s melting point is 13.4 °C, the trans isomer melts at 43 °C. The reason for this is that the trans isomer is straighter, packs better, and hence – having a much higher melting point.
The trans isomers have lower densities than their cis counterparts. In acyclic systems, trans isomers are more stable than cis isomers. In general, cis isomers have higher solubility in inert solvents.
Differences: Cis and Trans
In chemistry, the terms "cis" and "trans" are used to describe the orientation of atoms or groups of atoms in a molecule with respect to each other. This orientation can affect the physical and chemical properties of the molecule, such as its boiling point, melting point, and reactivity. The orientation of the atoms or groups of atoms in a molecule can be determined by the presence of a double bond, which can be either a cis or trans configuration.
Cis and trans refer to the arrangement of substituents on a double bond. In a cis configuration, the substituents are on the same side of the double bond, while in a trans configuration, they are on opposite sides. For example, in the molecule ethene (C2H4), there is a double bond between the two carbon atoms. In the cis configuration, the two hydrogen atoms are on the same side of the double bond, while in the trans configuration, the two hydrogen atoms are on opposite sides of the double bond. The cis configuration is often more polar than the trans configuration due to the orientation of the substituents.
In addition to the orientation of atoms or groups of atoms in a molecule, cis and trans are also used to describe the orientation of molecules in space. For example, in a cis configuration, two molecules or groups of molecules are on the same side of a plane, while in a trans configuration, they are on opposite sides of a plane. This orientation can affect the interactions between molecules, such as intermolecular forces and steric hindrance.
In social studies, the terms "cis" and "trans" are used to describe the gender identity of individuals. Gender identity refers to a person's internal sense of being male, female, or something else. Cisgender (often shortened to "cis") refers to individuals whose gender identity matches the sex they were assigned at birth, while transgender (often shortened to "trans") refers to individuals whose gender identity does not match the sex they were assigned at birth.
The concept of cisgender is often used in discussions of privilege and oppression related to gender identity. Cisgender individuals may have certain privileges or advantages over transgender individuals, such as not experiencing discrimination or violence based on their gender identity.
Transgender individuals may face a range of challenges related to their gender identity, including discrimination, harassment, and violence. In recent years, there has been increased awareness and acceptance of transgender individuals and their experiences, but there is still much work to be done to ensure that they are treated with respect and dignity.
The following table highlights the major differences between Cis and Trans −
Characteristics | Cis | Trans |
|---|---|---|
| Definition | The prefix “cis” is derived from Latin. It means “on the same side”. In the cis isomer, the substituent groups are placed on one side of a double bond plane or a non-aromatic cycle. | The prefix “trans” is derived from Latin. It means “on opposing sides”. In the trans isomer, the substituent groups are placed on different sides of a double bond plane or a non-aromatic cycle. |
| Polarity and boiling poin | Cis: The polarity causes increased intermolecular forces, which result in an increase of the boiling point. The cis isomers, which are more polar than the trans isomers, have a higher boiling point. | Trans: The trans isomers are less polar and have a lower boiling point than the cis isomers. The difference is more significant in substances with polar bonds. |
| Symmetry and melting point | Cis: The cis isomers are less symmetrical and have a lower melting point, compared to the trans isomers. | Trans: The trans isomers have higher symmetry and a higher melting point, compared to the cis isomers. |
Conclusion
In conclusion, the terms "cis" and "trans" are used in different fields to describe different aspects of objects, molecules, and people. In chemistry, these terms refer to the orientation of atoms or groups of atoms in a molecule, while in social studies, they refer to the gender identity of individuals.
Bond Parameters
Introduction
A variety of factors are used to evaluate covalent bonding. Bond length, bond strength, bond polarity, and bond multiplicity are only a few of them. Let's have a look at what these characteristics signify and how they affect us. Let's start with the bond length.
Bond Length
The equilibrium distance between the nuclei of two bound atoms in a molecule is defined as bond length. The bond length is determined by each atom in the bonded pair. Each atom of the bonded pair participates in the bond length of a covalent bond. The contribution of each atom is referred to as the atom's covalent radius. In a bonded condition, the covalent radius is calculated as the radius of an atom's core in contact with the core of a neighbouring atom.

is the formula for the bond length in a covalent molecule AB, where r_A and r_B are the covalent radii of two atoms and R is the bond length. The covalent radius is defined as half the distance between two comparable atoms in the same molecule linked by a covalent bond. In a non-bonded condition, the van der Waals radius indicates the entire size of the atom, which includes its valence shell.
Furthermore, in a solid, the van der Waals radius is half the distance between two identical atoms in separate molecules. Covalent radii are always bigger than van der Waals radii.
Factors influencing bond length include -
Because the distance between the valence shell electrons and the nucleus increases with the addition of electrons, bond length expands with the size of the atom.
For example,
Bond length reduces as bond multiplicity increases.
Bond Angle
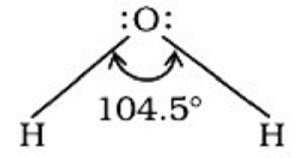
The angle between the orbitals having bonding electron pairs around the central atom in a complex or molecule ion is defined as the Bond Angle. Bond angle is measured in degrees/minutes/seconds and may be obtained experimentally using spectroscopic techniques. It helps us determine the shape of a molecule/complex ion by giving us a sense of the arrangement of orbitals around the central atom.
In water, for example, the H–O–H bond angle can be expressed as follows −
Bond Order
The number of bonds between the two atoms in a molecule determines the Bond Order in the Lewis theory of covalent bonds.
For example, let us consider H2 which has one shared pair of electrons, O2 having two shared pair of electrons and N2 having three shared pairs of electrons, the bond order would be 1, 2 and 3, respectively. The bond order is also three in CO wherein there are three shared electron pairs between C and O.
Bond orders are equal for isoelectronic molecules and ions; for example, F2 and have bond order 1. The bond order of N2, CO, and NO+ is 3.
Bond enthalpy increases as bond order increases, but bond length decreases. It is important for understanding the stabilities of compounds.
Bond Enthalpy
It's the amount of energy needed to break one mole of a specific sort of bond between two atoms in a gaseous state. Bond enthalpy is measured in kJ mol–1. The enthalpy of the H–H bond in a hydrogen molecule, for example, is 435.8 kJ mol–1.
The two factors affecting bond enthalpy are summarized below −
Bond Length
The greater the value of bond enthalpy, the shorter the bond length. The length of a C-C bond is 154 pm, which is longer than the length of a C=C bond, which is 134 pm. As a result, the bond dissociation enthalpy of the C-C bond is 433 kJ/mol, whereas the bond dissociation enthalpy of the C=C bond is 619 kJ/mol.
Atomic Size
The stronger the bond is, the smaller the bound atoms are. As a result, the bond dissociation enthalpy has to have a higher value. The bond dissociation enthalpy of the H-H bond, for example, is 435.8 kJ/mol, which is higher than that of the Cl-Cl bond, which is 243.5 kJ/mol.
The greater the bond dissociation enthalpy, the stronger the bond in the molecule will be. We have a heteronuclear diatomic molecule like HCl.
The determination of bond strength in polyatomic compounds is more difficult. The enthalpy required to break the two O – H bonds in the molecule, for example, is not the same. The difference in the value indicates that the second O – H bond changes as the chemical environment changes.
Conclusion
While a single covalent bond is created when two atoms share an electron pair, multiple bonds are formed when two or three electron pairs are shared. Some bound atoms have extra pairs of electrons not involved in bonding.
Lone pairs of electrons are what they're called. The arrangement of bound pairs and lone pairs surrounding each atom in a molecule is shown in a Lewis dot structure. Bond length, bond angle, bond enthalpy and bond order, are all important factors connected with chemical bonds that have a substantial impact on the properties of compounds.
FAQs
Q1. Mention some of the bond parameters effecting chemical bond.
Ans. The bond parameters affecting chemical bond are
Bond Length
Bond Order
Bond Angle
Bond Enthalpy.
Q2. Why Multiple Bonds are Stronger than Single Bonds?
Ans. As the quantity of bonding electrons between two atoms increases, the length of the bond between them shortens, resulting in the development of multiple bonds. The greater electron density between the atoms causes a stronger interaction between them, resulting in a decrease in bond length. The stronger the interaction, the stronger the bond. As a result, multiple bonds are more powerful than single ones.
Q3. What do you mean by the term Bond Length?
Ans. Bond length or bond distance is the distance between the centres of covalently bound atoms. X-ray diffraction of materials, electron diffraction, and spectroscopic (analysis of light absorbed/emitted by molecules) techniques are used to estimate bond lengths.
Q4. What is Van Der Waals Radii of Atoms?
Ans. In a non-bonded condition, the van der Waals radius indicates the entire size of the atom, which comprises its valence shell. Furthermore, in a solid, the van der Waals radius is half the distance between two identical atoms in separate molecules. Covalent radii are always smaller than van der Waals radii.
Q5. What is the trend followed by Single Covalent Radii in a Periodic Table?
Ans. It has been observed that single covalent radii increase down the group but decreases left to right along the period.
Experiments On Properties Of Acids And Bases
Introduction
Experiments on properties of acids and bases are one of the most vital topics in chemistry. It is discussed that the sour chemicals usually turns blue litmus into red are called acids. In a similar fashion, bases that are bitter tasting convert red litmus into blue. And acids and bases on reaction with one another give salt and water. This indicates neutralizing i.e, both the base and acid neutralize into the pH of water.
These facts will only remain assumptions if not tested and experimented with. Therefore, this article will consist of Experiments on Properties of Acids and Bases. There will be three experiments each with HCl as the acid and NaOH as the base. These will be tested with compounds like; a litmus solution (blue/red), zinc metal, and Sodium carbonate (solid).
Materials Required for Experiments on Properties of Acids and Bases
There are various materials that are required for conducting the Experiments on Properties of Acids and Bases. These are
Matchbox,
Dropper,
Test Tube stand,
Test tube holder
Burner
Thistle funnel
Litmus Solution or Paper (Both Red and Blue)
Test Tubes
Flat Bottom flask
Zinc Granules
Lime Water (Freshly Made)
Breaker
Sodium Carbonate (Solid)
Dilute NaOH
Dilute HCl

Figure 1: List of required chemicals and materials
(Nefronus, Zinc sample, CC0 1.0) (Ondřej Mangl, Uhličitan sodný, marked as public domain, more details on Wikimedia Commons) (Walkerma at en.wikipedia, Hydrochloric acid 04, marked as public domain, more details on Wikimedia Commons) (Ondřej Mangl, Hydroxid sodný, marked as public domain, more details on Wikimedia Commons) (Deamit, Одредување рН на примерок вода со Лакмусова хартија, CC BY 4.0)
What are Acids?

Figure 2: Common mineral acids and acetic acid
The compounds that when dissolved in water, result in yielding positively charged hydronium ions are termed as acids.
Eg. - releases and ions just like HCl releases and ions.
Experiments On Properties of Acids
Experiment 1: Litmus Test
Take two test tubes and place them on the stand for test tubes.
Now, label the two test tubes as 1 and 2.
Take the test tube 1 and fill in 5ml of the blue litmus solution.
Now pour 5 ml of red litmus solution in test tube 2.
Now, take HCl in a dropper and fill equal amounts in both test tube 1 and 2.
Look closely for the colour in the test tubes to change.

Figure 3: Litmus test
Deamit, Одредување рН на примерок вода со Лакмусова хартија, CC BY 4.0
Experiment 2: Sodium Carbonate (Solid)
Take a flat bottomed flask and add in Sodium Carbonate (1 gram) and a little distilled water.
Now take a cork (dry double bore). This cork should fit a thistle funnel. Also, a delivery tube should be fitted to it.
Now, use the dry double bore cork to close Flat Flask's mouth. Later add HCl gas (2ml).
The gas released is odorless and colourless. This gas then passes through the lime water and delivery tube.
On passing through the lime water, the lime water starts appearing milky.
The reaction involved in this experiment is:
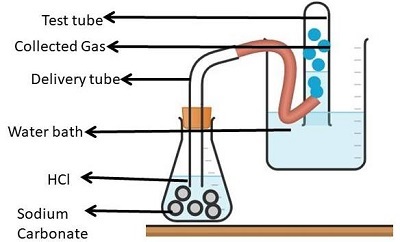
Figure 4: Action of Sodium Carbonate on HCl acid
Experiment 3: Zinc
Take a test tube (clean and dry) and put in it, Zinc granules.
Pour HCl (liquid) into the test tube and let the Zinc granules be submerged in it.
Now tilt the test tube a little and fix it with a cork properly.
Fix a Bunsen burner on the test tube and then light it.
Now close the test tube's mouth tightly. This should ensure that no vapours escape.
After leaving the solution for 2-3 minutes, it'll burn sounding robust and release a gas that's odourless and colourless.
Now, if you burn a matchstick in front of the test tube's mouth, a flame that's pale blue is seen followed by pop sound.
Below is the reaction involved in the above experiment:
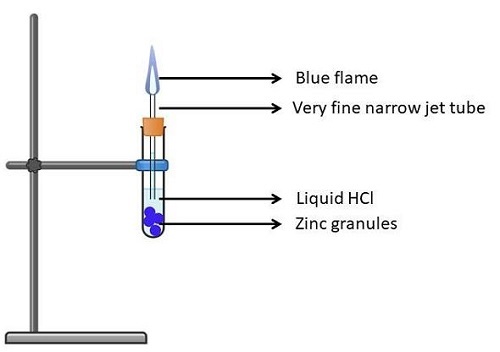
Figure 5: Action of Zn on HCl acid
What are Bases?
Figure 6: Strong and weak bases (colors are used for distinction from one another not by any scientific mean)
The compounds that when dissolved in water, result in yielding negatively charged hydroxide ions are termed as bases.
Eg- NaOH releases Na+ and OH- ions.
Experiments On Properties of Bases
Experiment 1: Litmus Test
Take two test tubes and place them on the stand for test tubes.
Now, label the two test tubes as 1 and 2.
Take the test tube 1 and fill in 5ml of the red litmus solution.
Now pour 5 ml of red litmus solution in test tube 2.
Now, take NaOH in a dropper and fill equal amounts in both test tube 1 and 2.
Look closely for the colour in the test tubes to change.
Experiment 2: Sodium Carbonate (Solid)
Take a flat bottomed flask and add in Sodium Carbonate (1 gram) and a little distilled water.
Now take a cork (dry double bore). This cork should fit a thistle funnel. Also, a delivery tube should be fitted to it.
Now, use the dry double bore cork to close Flat Flask's mouth. Later add dil NaOH (liquid).
There will be no changes i.e, no reaction occurs.
Experiment 3: Zinc
Take a test tube (clean and dry) and put in it, Zinc granules.
Pour NaOH (liquid) into the test tube and let the Zinc granules be submerged in it.
Now tilt the test tube a little and fix it with a cork properly.
Fix a Bunsen burner on the test tube and then light it.
Now close the test tube's mouth tightly. This should ensure that no vapours escape.
After leaving the solution for 2-3 minutes, it'll burn sounding robust and release a gas that's odourless and colourless.
Now, if you burn a matchstick in front of the test tube's mouth, a flame that's pale blue is seen followed by pop sound.
Below is the reaction involved in the above experiment −
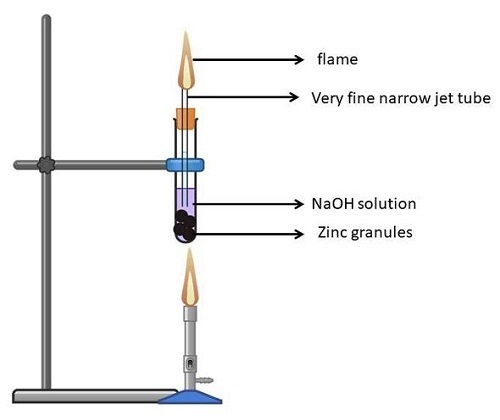
Figure 7: Action of Zn on NaOH
Precautions
Because hydrogen produced in large amounts results in explosion then you should ensure taking small quantities of reactants (NaOH, HCl, Zn).
Use a fine jet for hydrogen burning.
Only after your apparatus is carefully made airtight, you should add HCl to Sodium Carbonate.
Handling NaOH and HCl like chemicals should be done carefully because they can cause injuries.
Observations
| Experiment | Acids | Bases |
|---|---|---|
| Experiment 1: Litmus Test | The blue litmus solution turns into red when reacted with Hydrochloric Acid. | The red litmus solution turns into blue when reacted with NaOH. |
| Experiment 2: Sodium Carbonate (Solid) | is released on reaction of HCl with sodium carbonate. | No reaction is observed on reaction of NaOH with sodium carbonate. |
| Experiment 3: Zinc | and gas are the products as a result of the reaction between HCl and Zinc. | Sodium Zincate and gas are the products as a result of the reaction between NaOH and Zinc. |
Conclusion
On an experiment of Hydrochloric Acid with a blue litmus solution, the solution turns red. Similarly, in reaction with Sodium Carbonate, is released. Also, as HCl is reacted with Zinc it results in release of and gas. And therefore the acidic property of HCl is proven.
In a similar fashion, NaOH on reaction with red litmus solution turns it blue. And when reacted with Sodium Carbonate, NaOH has no reaction. Finally on NaOH reacting with Zinc, products formed are: Sodium Zincate and gas. Therefore, NaOH exhibits all the properties of a base.
FAQs
Q1. How can you experimentally identify acids and bases?
Ans. There are various experiments to identify bases and acids. One of which is the litmus test. Using a blue litmus solution you can identify acid and with the red litmus solution the bases. Because, blue litmus turns red on reaction with an acid and red litmus turns blue on reaction with a base.
Q2. What is the importance of base and acid indicators?
Ans. To identify a reaction's end point in a titration experiment, the Acid-base indicators are useful. These are also helpful whole gauging the pH values for different compounds. Also, the intriguing colour change demonstrations in science are also facilitated by Acid-base indicators.
Q3. Is there a possibility of a pH value in negative?
Ans. Although the scale for pH values only covers values between 0-14, the negative pH calculation is possible. In an acid with Hydrogen ion molarity to be greater than the normality of 1N, there is a possibility of negative pH value.
Examples of Bases
Introduction
Examples of bases with its properties are as follows −
Bases have pH value more than 7.
Aqueous solution of basic compounds undergoes ionization and can conduct electricity.
On reacting base with acids can produce salts.
The concentrated base or strong base is known as Caustic.
If base added to alkaline solution it has bitter taste.
Base get identify easily due to the presence of OH- ions.
On applying red litmus paper to basic solution it changes the colour into blue.
On adding to acids compounds base can react vigorously.
On adding base to water it can conduct electricity.
It is a slippery substance in nature.
Classification and Types of Bases
The basic compounds get classified on the basis of its concentration, acidity and ionization degree.
There are three types of bases as per its acidity −
Monoacidic base – The compounds has only one hydroxide ion (OH-) and able to react with only one hydrogen (H+) ion. Examples: NaOH, KOH, etc.
Di-acidic base – The compounds has two hydroxide (OH-) ions and able to react with two hydrogen (H+) ions. Examples: .
Tri-acidic base: The compounds has three hydroxide (OH-) ion and can reacts with three hydrogen (H+) ions. Examples: .
Types of bases according to its concentration −
Dilute base: Low concentration of base in solution. Example: Dilute KOH, dilute , etc.
Concentrated base: High concentration of base in solution. Example: Concentrated NaOH, concentrated , etc.
Types of base on it ionization degree −
Strong Base: The base which gets completely ionized in water solution is called strong base. Example: NaOH.
Weak Base: The base which does not completely get ionized into water solution is called weak base. Example: .
Uses of Bases
Base like NaOH (sodium hydroxide) is used in preparation of rayon, paper, soap, etc.
(magnesium hydroxide) is used as an antacid.
(calcium hydroxide) is used as a dry powder mixture for painting and decoration. It is also used in bleaching powder.
(ammonium hydroxide) is used in laboratories.
Examples of Bases
The examples of the various bases are listed below.
– Barium Hydroxide
is a strong base. It is a di-acidic base. It can ionize completely on adding to water and can form OH- ion in water.
– Ammonium Hydroxide
is a weak base. It is a monoacidic base. It cannot completely ionize on adding to water and form very less OH- ions in water solution.
NaOH – Sodium Hydroxide
NaOH is a strong base. It is a monoacidic base. It get completely dissociate into water. It is also known as caustic soda.
– Aluminium Oxide
can act both as acid and base as it is an amphoteric oxide. It can react with strong acid and form salt. Thus it acts like a base.
CaO – Calcium Oxide
CaO is a strong base. It comes under solid bases. It is a metallic oxide. On reacting CaO with water solution it forms a strong base. It is also known as quicklime.
Figure 1: Strong bases with aqueous solution
– Aluminium Hydroxide
is an amphoteric substance and acts as an acid as well as base. It acts as a weak base. It can react with strong acids.
KOH – Potassium Hydroxide
KOH is a strong base because it can dissociate totally in water solution. It is monoacidic base and also known as caustic potash.
– Ammonia
Ammonia can act as a lewis base as it can donate its extra electrons to lewis acid and shows basic character.
– Iron Hydroxide
is tri-acidic base. It acts as weak base as it get dissociate incompletely on adding to water solution.
– Strontium Hydroxide
The is a strong base. The hydroxides of group 2nd elements like Sr, Ba and Ca can form strong base.
– Copper hydroxide
is a weak base. It dissociate in very small amount on adding to water. Mostly of part is insoluble.
BaO – Barium Oxide
BaO is a strong base because it act as a very good proton acceptor.
– Calcium Hydroxide
is a strong di-acidic base. It can completely ionize in water. It is also known as slaked lime.
– Lead Hydroxide
is a weak base as it is rarely soluble in water and form ions in weak acidic solution.
MgO – Magnesium Oxide
MgO act as an basic oxide as it can produce basic Mg(OH)2 on mixing in water.
– Magnesium Hydroxide
is a strong and di-acidic base. It gets ionized 100% in water solution.
RbOH – Rubidium Hydroxide
RbOH is a monoacidic base. It is a strong base due to it 100% ionization in water.
BeO – Berrilium Oxide
BeO is an amphoteric compound. It can act both as an acid and a base.
– Zinc Hydroxide
is a amphoteric substance. It can act both as acid and a base. On reaction with acid it can act as a base.
NaH – sodium Hydride
NaH is a strong base and dissociate completely on adding to water solution.
CsOH – Cesium Hydroxide
CsOH is a strong base. It is a monacidic base. Cesium is more electronegative due to which it act as strong base.

Figure 2: Weak bases
Conclusion
Basic compounds are the compounds which can accept protons from other compounds. Bases are differentiate as Lewis base, Arrhenius base or Bronsted-Lowry base. Basic solutions generally show pH value more than 7.
There are various types of bases like monoacidic, di-acidic, tri-acididc, etc. the strong base is that which can completely dissociate in water. The weak bases are those which get incompletely dissociates in water solution. CsOH, NaOH, RbOH, , etc. are the examples of strong bases. , etc. are examples of weak bases.
FAQs
Q1. How could you determine that the compound is basic in nature?
Ans. If the compound shows the ability to accept protons from other compound it shows a basic nature. Also basic compound is determined due to formation of hydroxide ions in aqueous solution.
Q2. What is di-acidic base?
Ans. The compounds which contain two hydroxide ions and can form bonds with two hydrogen ions are called di-acidic base.
Q3. What happen when basic compound added to water?
Ans. When basic compounds get added to water it get dissociates into ions and if OH- ions form it confirms its basic nature.
Q4. CsOH is a strong or weak base?
Ans. CsOH is a strong base because it can completely dissociate in water and form more OH- ions.
Q5. What is lewis base?
Ans. Lewis base is a species which can donates its electrons to a lewis acid.
Fe3o4 Iron Oxide
Introduction
(Iron oxide) is one of the easily synthesized compounds occurring in nature are iron oxides. The iron oxides are better known as magnetic oxides have been in use by humans for ages now. A very common example of magnetic oxides being used are the nanoparticles of iron oxide (IONPS). For around 50 years, they have been used for diagnosis conducted in vitro as a contrast agent. Iron oxides have the formula as . They are also simply seen as rust.
What is Iron Oxide?
Laboratory-easy synthesized compounds, also commonly found in nature are Iron oxides. The iron oxides existing in nature are 16 in number. They include;
Oxides of iron
Hydroxides of iron
Hydroxy-Oxides of iron
They are usually found to have formed as a result of reactions. These reactions are usually aqueous. They show variability in pH levels and redox levels. The oxides have a common basic composition. It includes; Fe (Iron), O (Oxygen) or OH (Hydroxide). They are differentiated among themselves by varying valency and crystal structure. The common examples of iron oxides are; Hematite, Akaganeite, Magnetite, Lepidocrocite, and Goethite.
Iron Oxide (II, III) has the chemical formula . Mineral name for is magnetite. This is the most commonly found iron oxides found in nature. The name of iron oxide is usually followed with (II, III) because they have been found containing as well as ions. This explains 's attraction to even the smallest magnetic field (external).
Properties of

Figure 1: Magnetite (Fe3O4)
James St. John, Magnetite-pyrite-actinolite rock (Jurassic, 156-162 Ma; Mina 5, Marcona Magnetite Deposit, Ica Department, Peru) 1, CC BY 2.0
Following are the relevant properties of ,
The colour appears in is dark color most commonly black appearing.
231.531 g/mol is the molar mass of .
has a melting point of 1597°C.
2623°C is the melting point for .
It is found as an odorless and solid black powder when observed under room temperature.
The structure of commonly found is cubical and inverse spinel.
has a good electrical conductance. It is 106 times the conductivity of .
Proper induction in a magnetic field helps the particles to act magnetic, resembling tiny magnets.
The compound has also been called Mars Black, a black pigment.
The Harber process is catalysed using as the catalyst. It helps produce Ammonia.
MRI scanning is facilitated with the use of - nanoparticles. They play the role of contrast agent.
Uses of Iron Oxide
Like any other metal oxide in nature, iron oxide too has significant uses. They have been mentioned below,
Black iron oxide (ordinary) is utilised for inks used in die stamping and copperplate.
Among different components used for plastic, pharmaceuticals, inks, and paint industry products, the most common is iron oxide.
Salts of copper oxide are very often used in wastewater treatment, fertilizers, dying of textiles and production of additives for feed.
Copper oxide also is very popular as a polishing material in the jewellery industry.
Nanoparticle Synthesis
The nanoparticles like; maghemite and magnetite are utilised very often in colloidal suspension preparation. They are now synthesised using solution combustion. The process in concluded in the following steps;
A flask with a round bottom is taken.
The flask is used to store a solution of,
fuel utilised in magnetite synthesis and
fuel utilised in maghemite synthesis
Heat the solution to 400°C without the presence of air.
Water evaporates, resulting in a combustion reaction (smouldering), as a result a black powder is left behind.
Black powder is now treated with distilled water after crushing.
This washed black powder is exposed to 80°C and dried.
This powder is finally treated with H2O2 and any carbon remaining as a residue (as a result of the earlier combustion reaction) on the surface is removed.
The nanoparticles are obtained.
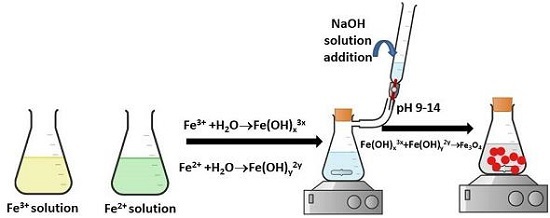
Figure 2: Preparation of Iron Oxide Nanoparticles
Iron Oxide Nanoparticles (IONPs): Properties
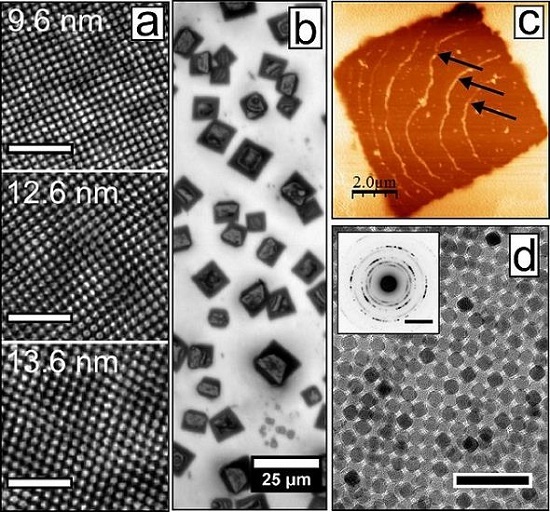
Figure 3: Iron Oxide Nanoparticles
Erik Wetterskog et al., Self-assembly of iron oxide nanocrystals, CC BY 3.0
Some important points about Iron Oxide Nanoparticles are,
They are usually a product of (Maghemite) or (Magnetite) nanoparticles.
The diameter for these nanoparticles lies in a range of 1 to 100 nanometers.
The use of these nanoparticles is mostly found in drug delivery, data storage (magnetic), Biosensing, etc.
There is a significant increase seen in the ratio of the area to volume of these nanoparticles. Therefore, in turn increasing their binding capacity significantly higher. They also show an excellent dispersion rate in a solution.
Another one of their qualities is supermagnetism. This can be seen in NPs with sizes ranging from 2 to 20 nanometers. This indicates towards; zero magnetism when they are found outside the presence of an external magnetic source or field. This makes Nanoparticles highly stable in solutions.
Conclusion
The chemical compounds made of both iron and oxygen as its constituents are called Iron oxides. There are 16 iron oxides/oxyhydroxides known to men at present. The most common among the iron oxides is rust. They are very easily available in nature. With being easily accessible in nature, they can also be with-ease synthesised within a laboratory.
The magnetite and maghemite nanoparticles using synthesis named solution combustion use iron oxides as an important component of that solution. These Nanoparticles later help to prepare colloidal suspensions.
Ordinarily available black iron oxide also helps in preparing, copperplate and inks (die stamping). The most common industries that popularly utilize iron oxides are; cosmetic, paint, plastic, ink, and pharmaceuticals.
FAQs
Q1. What is the role of magnetite in iron oxide nanoparticles formation?
Ans. Magnetite plays the role of an important component of the solution undergoing combustion to form iron oxide nanoparticles.
Q2. What is understood by iron oxides?
Ans. Iron oxides are the oxides of transition metals. They can vary on the basis of stoichiometry and crystals. Among some very common iron oxides are Fe2O3 (Maghemite) and Fe3O4 (Magnetite).
Q3. What are some industrial uses of magnetite ()?
Ans. The industrial uses of iron oxides are in the field of; paint, ink, pharmaceuticals, cosmetics, etc. They are also utilized in preparation of IONPs.
Q4. What are some commonly known properties of iron oxide nanoparticles?
Ans. The properties of iron oxide NPs are,
Are highly stable in solutions as they show super magnetism.
Have a higher binding capacity.
Help in storage of magnetic data.
Have a higher dispersion rate in a solution.
Evaporation Causes Cooling
Introduction
Evaporation causes cooling which is based on evaporation of a liquid from any surface leaving a cooling effect. The degree of cooling effect are left by the evaporating liquid can vary based on the liquid. It is exemplified by evaporating alcohol or water that leaves a cooling effect on the surface.
The variation in degree of cooling is also very clear between alcohol and water. Where, alcohol has a higher degree of evaporation of cooling effect than water. This variation depends on the nature of molecules of the liquid.
What is Evaporation?
A very common definition for evaporation is the process of conversion of a liquid into vapour is called evaporation.
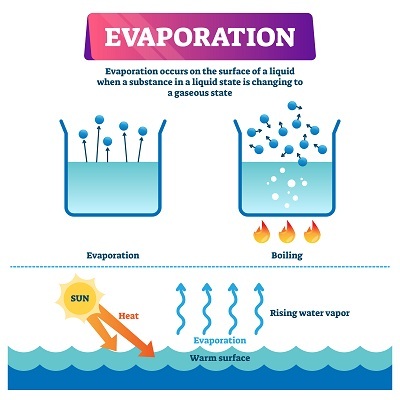
Figure 1: Process of Evaporation
Any liquid or solid being converted into vapour form results in evaporation and simultaneously a cooling effect is felt. The best example to understand cooling through evaporation is spraying perfume and experiencing cold feeling. This signifies the transformation of the liquid from the perfume bottle to vapour during spraying it. One can only differentiate the degree of cold experienced in different liquids while evaporating.
Evaporation causes Cooling
Evaporation causes cooling can be understood by the cooling effect on water on earthen pots. It can be observed the temperature of stored water in earthen pots is lower than the room temperature. The primary reason for cool water in earthen pots is the seeping of water through its microscopic pores. The evaporating water through pores absorbs the heat inside the pot resulting in water cooling.
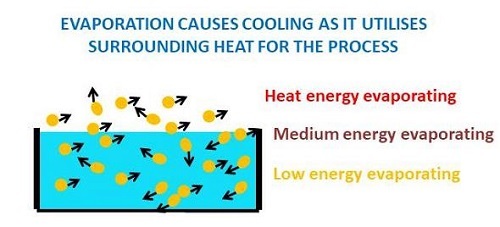
Figure 2: Cooling as a result of Evaporation
This cool water helps to keep refresh in the summer season. As the rate of evaporation is affected by temperature, the cooling of water slows down during rains and speeds up during summers.
Factors affecting the rate of Evaporation
Every other natural phenomenon is affected by different factors. The rate usually is determined by the role of these factors. Similarly, there are factors affecting the rate of evaporation. These are as follows −
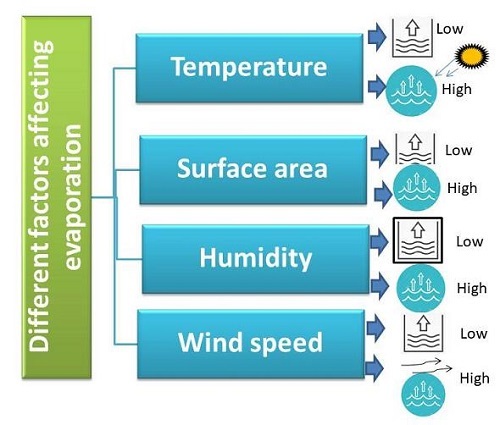
Figure 3: Factors affecting Rate of Evaporation
Temperature
Temperature (around the substance) is a factor that stands directly proportional to the rate of evaporation. With the increase in temperature the rate of evaporation also increases and vice-versa.
Atmosphere's Humidity
The evaporation rate stands inversely proportional to the humidity of the atmosphere around. This means lower the humidity higher is the rate of evaporation.
Surface Area
Evaporation is a surface phenomenon. Evaporation rate increases with the increase in surface area of the given liquid. Therefore, more the surface area more is the rate of evaporation.
Force of Attraction (Intermolecular)
Intermolecular forces become a very important factor among deciding factor for evaporation to take place. Rate of evaporation or even the very initiation of evaporation is determined by the intermolecular forces of attraction. The forces are different for different liquids. More the forces between molecules, lesser are the chances of evaporation.
Wind's Speed
The speed of wind is directly proportional to the evaporation rate. This is exemplified by quick drying of clothes witnessed on a windy day.
Evaporation: Application
Some of the common and overlooked applications of cooling effect of evaporation are as follows −
Cotton clothing worn in summers. It is to let the clothes absorb water in the form of sweat. This leads to them acting as a coolant or cooler themselves.
Drying and concentrating samples for laboratory purposes is another application of evaporative cooling. This is usually used in chromatography and spectroscopy.
Matki, a traditional container in Indian households, also works on the principle that evaporation causes cooling.
Air conditioners based on evaporative cooling provide cool air by blowing air through a filter soaked in water.
Sweating, a natural phenomenon based on sweat evaporating offs the body, leaving a cooling effect.
Evaporation Causes Cooling: Advantages and Disadvantages
Advantages
The advantages related to evaporation causes cooling are as follows −
It is the ideal situation for cooling in a dry climate. The lower humidity in the air (dry air) propagates the rate of evaporation thus cooling effect is increased.
An evaporative cooler when used can also be effective in filtering out the allergens, dust particles, and pollens in the air.
Evaporative cooling can be a method of enjoying convenience with managing health and avoiding any harm to nature.
Using evaporative cooling helps you reduce energy wastage by 80%.
Disadvantages
The disadvantages accompany the phenomenon of evaporation causes cooling are as follows −
Evaporative cooling is a risky choice for high humidity areas. This makes this less desirable in comparison to air conditioners not using this phenomenon as its core.
It heightened humidity due to the evaporative cooling impacts a negative sign. It promotes growth of mildew, dust mites etc. This plays a role in triggering asthma and various different allergic complications.
Conclusion
The phenomenon of evaporation causing cooling is a property of any liquid. The liquid evaporates after absorbing energy in the form of heat. The heat absorbed helps in evaporating the water molecule in slow rate and makes thee environment cool as heat has been absorbed by the water molecules.
This is how evaporation causes cooling. This phenomenon is applied in various daily needs. Clothing, appliances, etc. are some very common examples of applications of evaporation causing cooling in daily needs.
FAQs
Q1. What is condensation?
Ans. Condensation can simply be defined as a process opposite to evaporation. Condensation undergoes loss of heat and the vapours of water turn into droplets of water. This process is called condensation.
Q2. Is evaporation an endothermic or exothermic process?
Ans. As evaporation includes absorption of heat, it is an endothermic process.
Q3. In context with evaporation, what does surface phenomenon mean?
Ans. When the surface molecules of any liquid convert into gas it results in evaporation. This makes evaporation a surface phenomenon because surface molecules are involved.
Q4. What is the role of evaporation causes cooling in cooling down a room's temperature?
Ans. The water on being pumped in the machine pushes out or forces our air through the outlet. This air is cooler because the water absorbs the heat. This causes cooling down of the room temperature. This water on absorbing heat finally evaporates as water vapour.
Factors Affecting Solubility
Introduction
Factors affecting solubility are mentioned below as follows −
Solutes: The substance which is added to the solvent to dissolve into it.
Solvents: The liquid part or major volume part which absorbs the solute particles when added to it.
Insoluble: If some stones or sand are added into water and tried to mix it, these solute particles are not dissolved into water hence, the solute (stone or sand) is insoluble in solvent water.
Soluble: If a tablespoon of honey or sugar is added into water and tried to mix it, it completely dissolve into water and hence, the solute (honey or sugar) is soluble in solvent water.
Sparingly soluble: If some substance like, oil or salts like silver chloride are added and mixed into water, it get dissolve in very small amount hence, the solute (oil or ) is sparingly soluble in solvent water.

Figure 1: Comparative soluble, sparingly soluble, and insoluble compounds in solvent
ZabMilenko: orginal ZooFari: vector Mrmw: optimized, multilang, Chemical precipitation diagram multilang, CC0 1.0
Factors Affecting Solubility
There are three main factors affects solubility of substance are as follows −
Temperature (affects solid and gases)
Pressure (affects only gases)
Surface area (rate of solubility)
Temperature a factor which affects solubility of Solids and Gases
Temperature mainly effects on the solubility of solid and gas compounds and there is no predictions mentioned on liquid solubility. Temperature effect on solubility of solid compound which depend on the reaction i.e. an exothermic or endothermic. To explain this Le-Chatelier’s principle prescribed some postulates −
Exothermic reaction (ΔH is negative or less than zero)
Temperature rise creates strain on reactant part due to extra heat applied. Le-Chatelier principle suggests that equilibrium shifts left (reactant side) increases the reactants and decreases the solubility of solid compounds.
Endothermic reaction (ΔH is positive or greater than zero)
Temperature rise creates strain on product side due to application of extra heat. Le-Chatelier principle suggests that equilibrium shifts right (product side) increases the products and increases the solubility of solid compounds.
Temperature can directly affect the solubility of substances. For ionic solid compounds, solubility increases with increase in temperature. With increase in temperature the solid particles dissolve fast in the solution, due to which the solute particles are more interacted with solvent particles hence, the solubility rate increases. With increasing temperature more quantity of solute particles is miscible in solvent i.e. more particles of solute are miscible at high temperatures.
If salt is added into water it gets readily soluble in water. But if we apply external heat to that solution, more quantity of salt is added to water. This happens because the intermolecular forces between solvent molecules increases as well as hydrogen bonds (in case of water solvent) get easily breaks with increasing temperature and the salt (solute) particles are accompanied in between the spaces of solvent molecules.
In case of gaseous compounds, solubility decreases with temperature rise. With rise in temperature, the gas molecules moves fast and hence get evaporated from liquid solvent. Thus, gaseous compounds solubility decreases with increase in temperature.
Pressure which affects Solubility of Gases
Pressure generally affects the solubility of gaseous compounds in liquid solvent and not for solid compounds. When external pressure is applied on gaseous substance on solvents surface, the gaseous particles are immersed and occupied some space into the liquid solvent. For instance, carbonated soda (Coca cola), external pressure is applied on carbon dioxide gas molecules to enter into the soda can. If the pressure of gas decreases, gas solubility also decreases. As we open the soda can the, pressure get decreases and the gas instantly leaves the solution and comes out of the can.
Solubility of gas pressure is prescribed in Henry’s Law states that, at given temperature, the solubility of gas in liquid solvent is proportional to its partial pressure of gas in liquid. For instance, Scuba diving, in a deep sea when person dives, more gases are soluble in blood with the rise of pressure at underwater and when person comes out of the sea all the soluble gases slowly releases out from blood.
Surface area a factor which affects Solubility Rate
The bigger the size or surface area of solute its solubility rate is low while small size or surface area of solute its solubility rate is high. For instance, sugar with large surface area or particles, it takes more time to dissolve into water, while salt or sugar with small surface area or particles takes less time to dissolve into water. Thus, the molecules with big surface area soluble for longer time while the molecules with fine particles can soluble quickly.
For instance the copper (II) sulphate has various forms like crystalline form with large crystals and fine amorphous powder form. If we take same amount of both form of copper (II) sulphate i.e. 5g to dissolve in 50mL of solvent water. The crystalline form of copper (II) sulphate takes more time to dissolve in water even it needs stirring and shaking for some time. While the fine amorphous form of copper (II) sulphate can get dissolve in very less time with few seconds of stirring. Thus, the particle size or surface area of solute affects the rate of solubility with different time.
Conclusion
There are three basic factors affects solubility i.e. temperature, pressure, and surface area of solutes. Temperature affects only solid and gaseous compounds. With the rise in temperature solubility of solids increases while rise in temperature results in a decrease in solubility of gases.
By applying external pressure on any solvent surface the gaseous solute get interacts with solvent particles and dissolved into the solvent. Surface area factor affects the rate of solubility of solute into solvent. Solute with large surface area takes more time to dissolve into solvent while solute with small surface area takes less time to dissolve into solvent.
FAQs
Q1. What are solutes?
Ans. Solutes are solid, liquid or gaseous substances added to solvent.
Q2. Which factors affects solubility?
Ans. Temperature, pressure and surface area are the factors affecting solubility of solid- liquid, liquid-liquid or gas-liquid solutions.
Q3. What happen when external temperature applied on solid solutes and liquid solvents?
Ans. When temperature applied to solid solute mixed with liquid solvent, the solubility of solid solute increases in solution.
Q4. What happen when external temperature applied on gaseous solutes and liquid solvents?
Ans. When temperature applied to gaseous solute mixed with liquid solvent, the solubility of gaseous solute decreases in solution.
Q5. How the pressure affects the solubility of gaseous solutes?
Ans. When pressure applied on solvent surface the intermolecular forces breaks and the gaseous particles get interacts with them and get dissolved into solvent.
Q6. What is the effect of surface area of solutes on solubility?
Ans. Bigger the surface area of solute decreases the rate of solubility. Lesser the surface area of solute, increase the solubility rate.
Examples of Gases
Introduction
Examples of gases are as follows: oxygen, hydrogen, helium etc. It is not a new phenomenon that gases are one of four states of matter. Gases are made up of molecules that are further made up of individual atoms. Those atoms may be the same or different thereby forming elements and compounds respectively. The properties associated with the gases lie between the liquid and plasma states.
All the physical characteristics of gases and the compositions are based on their intermolecular forces and intermolecular spaces between the constituents. Gases have very wide spaces between them due to the dominance of intermolecular separation over intermolecular forces as compared to liquid and solids. That is the reason why gases cannot be held or put into a shape like solids and liquids. All of these properties related to gases are governed by temperature and pressure which are inversely proportional to each other in the case of gases.
Figure 1: Description of Gas molecules
Examples of Gases
Gases exist in different types and have many examples. Examples of gases can be categorized in different contexts. The two categories in which examples of gases are categorized are −
Gases of Homoatomic molecules
Gases of Heteroatomic molecules
Gases of Homoatomic Molecules
Homoatomic gases as the name suggests are gases that consist of only a single type of atom and made up of the same element. These are also denoted as elemental gases. Homoatomic gases exist under standard temperature and pressure conditions. Due to changes in pressure and temperature, the gases can change their composition. Gases of homoatomic molecules are divided into three types depending upon the number of atoms involved.
Monatomic gases
Diatomic gases
Triatomic gases
Figure 2: Example of Homoatomic gases
Monatomic Gases
Monatomic gases are those homoatomic gases which are made up of single atom. The composition of monatomic gases is very simple in the context of thermodynamic properties due to the absence of rotational and vibrational constants and due to the involvement of a single element. The common examples of monatomic gases are the noble gases and vapors of certain metals. These are as follows −
Helium (He)
Neon (Ne)
Argon (Ar)
Krypton (Kr)
Xenon (Xe)
Radon (Rn)
Sodium vapor (Na)
Potassium vapor (K)
Diatomic Gases
Diatomic gases are evidenced from the name, which involves the bonding of two same or different elements. Under the category of homoatomic gases, these are considered homonuclear diatomic gases. Diatomic gases are usually made up of nonmetals and are formed through covalent bonding. Covalent bonding is a chemical bonding type that involves sharing of electrons.
In the case of homonuclear diatomic gases, the covalent compounds or gases formed are non-polar covalent gases. This is because the same atoms are bonded to each other which does not involve a difference in the charges and hence the absence of net dipole moment. Unlike monatomic gases, these are not simple and the change in temperature and pressure leads to changes in their composition as well. Diatomic gases are not considered as good conductors of electricity or heat. Examples of homonuclear diatomic gases are −
Hydrogen gas
Nitrogen gas
Oxygen gas
Fluorine gas
Chlorine gas
Bromine gas
Triatomic Gases
As the name suggests, triatomic gases comprises bonding between the three atoms. The definition is flexible and like diatomic gases, these may also be homonuclear and heteronuclear. Here, only the homonuclear part is discussed. It is very well known that the most common homonuclear triatomic gas comprises of three oxygen atoms. There are many other homonuclear triatomic gases as well but these are not stable and eventually break into cations or other stable products. So the stable homonuclear triatomic gas is −
Gases of Heteroatomic Molecules
Heteroatomic gases are made up of molecules of different atoms. Like homoatomic molecules, these are also divided into mono, di, and triatomic gases of heteronuclear origin. Heteroatomic gases are also called mixed gases as these are made up of different types of atoms. The bonding in heteroatomic gases is covalent, electrovalent, or dative. Hetero atomic gases depending upon its diversity in bonding and the type of atoms involved are classified into organic or inorganic gases. Various examples of heteroatomic gases of varied categories are mentioned below −
Air (Combination of oxygen, carbon dioxide, nitrogen, and other gases)
Carbon dioxide
Carbon monoxide
Acetylene
Butane
Dimethyl ether
Ammonia
Hydrogen sulfide
Methane or Natural gas
Nitrous Oxide
Ethane and many more.
Figure 3: Example of heteroatomic gases
Toxic Gases with examples
There are certain gases are not good for inhalation and may be dangerous and even deadly for the human body. Accidental breathing of toxic gases or even mild exposure can lead to the death of the person and in some cases; it could make a person feel light-headed and nauseous. People working in heavy industry or chemical manufacturing plants should be aware of the toxic gases and should take necessary measures while working. The list of toxic gases examples is discussed below −
Vinyl Chloride
Trichlorosilane
Trimethylamine
Sulfur dioxide
Ozone
Osmium tetroxide
Hydrogen chloride
Hydrogen Sulphide
Phosphine
Phosgene
Tungsten hexafluoride
Formaldehyde
Application of various Gases
There are a variety of examples of gases that are important in sustaining life and its working on earth. Gases are of a variety of types and can belong to different categories. The list of examples of gases is endless. The physical and chemical properties associated with gases and their behavior makes them useful in a variety of applications. Gases are found in the usage of a variety of environments and conditions.
The natural usages of gases which play the role of basic elements for survival on earth are discussed. Air is required for breathing. Various biogeochemical cycles which balance the ecosystem are fulfilled only with the existence of gases. The growth of crops and maintenance of food production are fulfilled by gases that have a good nitrogen component in them. For instance growth of leguminous plants is possible only by conversion of atmospheric nitrogen gas into nitrates which are further used by the bacteria for the proper growth of the plants.
In Industries, the gases are manufactured at a commercial scale to aid different processes and sectors. These gases are called Industrial gases. Both organic and inorganic industrial gases are used in a variety of industrial processes like steel making, petrochemical analysis, semiconductor industry, medical industry, fertilizer production, H2 analysis industry, etc.
Conclusion
In nutshell, gases are an integral part of our living and in the smooth functioning of life on earth. It is not of significance for only human life existence but also to maintain the desired ecological balance and sustainability. Commercially, gases are a boon for a variety of sectors and industries. Though we are not able to enclose it due to its properties, its existence, and usage cannot be denied.
FAQs
Q1. What are gas laws? Explain briefly all the gas laws.
Ans. Gas laws are the group of laws that look out for different behavioral changes in gases based on changes in their volume, pressure, temperature, and the number of moles of the gas. Four gas laws are established with the relation between all the factors responsible for the modification in gas’s behavior. These laws are −
Boyle’s Law: Establish the relationship between pressure and volume.
Gay Lussac’s Law: Exhibit the relationship between pressure and temperature.
Charles’s Law: Defines the relationship between volume and temperature
Avogadro’s Law: Define the relationship between volume and amount
Q2. Discuss briefly Ideal Gases.
Ans. An ideal gas is a theoretical concept that works under standard temperature and pressure conditions where the gases are not subjected to outside environmental conditions. The ideal gases follow the ideal gas equation
In the real world, no gas is an ideal gas but under certain standard pressure and temperature conditions, noble gases, N2, and O2 show ideal gas-like behavior for a certain time.
Q3. How is the pressure on the gas generated?
Ans. The pressure on the gas is generated when its molecules collide with each other and with the walls of the container. The collisions transfer a small amount of force and generate momentum. Momentum usually depends upon the speed which further depends on the temperature increase. Hence more the temperature increases, the more will be the speed and momentum and the more will be pressure exerted by the gases. This pressure can be used for various chemical and thermodynamic processes.
Q4. What role do gases play in photochemistry?
Ans. Gases are found in the atmosphere and hence, all its study is categorized under atmospheric chemistry. Photochemistry on the other hand is the use of light for various reactions which again happens in atmospheric conditions. So, gases aid in the photochemical reaction. For instance, chain reactions are observed with ozone and chlorofluorocarbons. In the ionosphere layer, gases usually convert into ions because of UV or cosmic radiation which are strong enough to remove electrons.
Fehling Test
Introduction
Fehling’s test is possible in presence of certain organic functional group with the help of following reacting agent as mentioned below −
Carbohydrates with free C=O (carbonyl) group i.e. aldehyde or ketones can possibly behave as a reducing sugars.
Fehling’s solution is mixture of , (strong alkali), and potassium sodium tartarate which is deep blue in colour.
On heating the test sample with Fehling’s solution, oxidation occurs by bistartarocuparate(II) complex which oxidises aldehydes into carboxylic acid.
Reduction occurs of copper(II) ion complex and forms red or yellow insoluble precipitate of [cuprous(I) oxide].
By oxidation of ketones can form short chain of acids.
When and NaOH react in the solution, tartrate ions inhibit insoluble formation by producing bistartarocuprate(II) complex.
These complexes inhibit the black cupric oxide formation and discharge cupric ions for reduction process.
Black cupric oxide precipitate forms on heating Fehling’s solution without reducing sugars.
Fehling’s Solutions
In laboratories, Fehling’s test is done by using freshly prepared solution known as Fehling’s solutions. Basically Fehling’s solution are available in two forms i.e. Fehling’s A solution and Fehling’s B solution.
Fehling’s A solution is composed of i.e. copper(II) sulphate which is in liquid form gives deep blue colour.
Fehling’s B solution is a mixture of Rochelle salt i.e. potassium sodium tartarate and sodium hydroxide (NaOH) which is a strong alkali. This is a transparent liquid solution.
Both Fehling A and Fehling B solutions are prepared separately in fresh form and afterwards it is stored. Later on, both of Fehling’s A and B solutions are mixed to prepare a absolute product of Fehling’s solution. While mixing this solution both Fehling A and B solutions are taken in same volume and hence, a deep blue colour solution is formed. The deep blue colour of the solution is due to the formation of ion of tartrate complex. Here, in the solution the tetra anions of tartrate behave as chelating agents.
Fehling’s test plays a vital role to distinguish the aldehydes and ketones compounds. If the test result is positive then it confirms the presence of aldehyde and if the test result is negative it indicates absence of aldehyde may be presence of ketone or any other functional group which does not respond to the Fehling’s test.
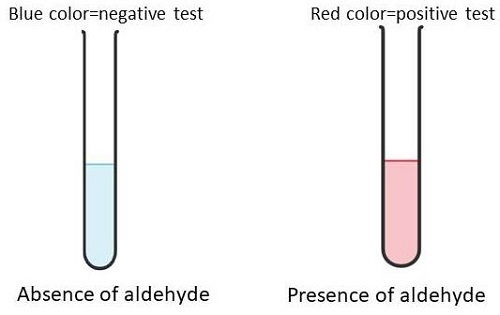
Figure 1: Indicator of Fehling test
Procedure
Fehling’s test is done with the following procedure −
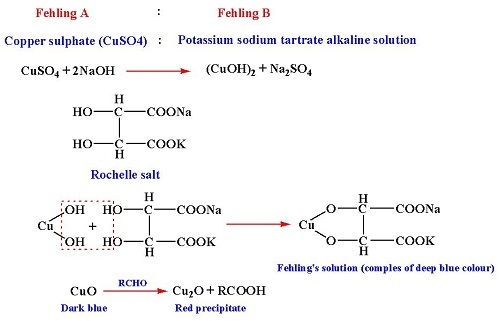
Figure 2: Fehling’s test
In an empty dry test tube take the test sample.if there is any formation of red precipitate.
In another test tube take distilled water as a blank.if there is any formation of red precipitate.
Then add the Fehling’s solution in both the test tubes containing sample and blank.if there is any formation of red precipitate.
Place the test tubes in water bath container on a burner to heat.if there is any formation of red precipitate.
Observe the colour change in the test tubes if there is any formation of red precipitate.
Results
After the experiment given above show some changes in the test tubes. The formation of brick red colour then it shows positive result and if there is no change in colour or it has only blue colour then it shows negative result.
If both the Fehling A and B solutions is mixed and heated. Fehling B solution behaves as a chelating agent as it is predominant in the solution. If the test sample solution contains aldehyde or sugar compound or any chelating particles, the colour changes to brick red on addition of Fehling’s solution. If the brick red precipitate occurs in the sample solution, it shows that aldehyde or reduced sugar group is predominant in it. Thus, the prime purpose to execute Fehling’s test is to determine the reduced sugar. For better results and accuracy in Fehling’s test, it is necessary to prepare fresh Fehling’s solutions every time before the further procedure of test. To the extent of Fehling’s test for the samples like fructose, lactose, glucose, etc. always shows positive sugar test.
Reaction of Fehling’s Test
The above reaction occurs between aldehyde and copper(II) ions, hence there is formation of RCOO- (carboxylic ion) ion.
Here, the reaction show colour change after addition of the tartrate complex to the previous reaction.
The above reactions are redox reactions, the copper(II) ions are reduced to copper(I) oxide on completion of redox reaction and hence, there is formation of brick red colour precipitation which is not soluble (insoluble) in water. The sodium (Na) salt of carboxylic acid remains as it is in that solution. Therefore, the brick red precipitate formation indicates the positive result of Fehling’s test.
Applications
Fehling’s test has various applications and used for many purposes, some of the applications are as follows −
Common use of Fehling’s test is to detect the presence of carbonyl group containing compounds i.e. aldehydes or ketones. Aldehydes are oxidised and shows positive test results while ketones (except α-hydroxy ketones) shows negative test results.
Fehling’s test also primarily is used to determine the simple sugars (monosaccharides) including other reducing sugars. Thus, if monosaccharides are present in both aldoses and ketoses shows positive results. Though ketoses get converts into aldoses due to presence of base in that reagent.
Diabetic patients undergo glucose urine test which is done by using Fehling’s test to identify the person is diabetic or not.
It is also used in the starch break down which converted into a syrup containing glucose and maltodextrin.
Fehling’s test also is used to identify formic acids with positive test result.
Conclusion
Fehling’s test is discovered by a German scientist H. C. Von Fehling. Fehling’s test is basically used to identify the carbonyl group containing compounds like aldehydes, ketones, carbohydrates, reduced sugars, etc. There are two types of Fehling solutions i.e. A and B. Fehling solutions are composed of only copper (II) sulphate, NaOH, and sodium-potassium tartrates.
If there is presence of any sugar, aldehyde or ketone group in the test sample then the solution colour changes from dark blue to brick red indicates positive result and if no colour change means absence of such functional group in the sample which results in no significant colour change and shows negative result.
FAQs
Q1. Who discovered Fehling’s test?
Ans. It is discovered by H. C. Von Fehling.
Q2. What is Fehling A solution?
Ans. The solution of copper (II) sulphate is known as Fehling A solution.
Q3. What components present in Fehling B solution?
Ans. Strong alkali i.e. sodium hydroxide and sodium potassium tartrate is present in Fehling B solution.
Q4. What results forms if the sample test compound present aldehyde?
Ans. It shows positive test when the deep blue colour changes to brick red precipitate confirms the presence of aldehyde.
Q5. Give three uses of Fehling’s test.
Ans.
It is used to distinguish between aldehyde and ketone groups.
It is used in glucose urine test for diabetic patients.
It is used to determine carbohydrates (simple sugar or monosaccharides).
Q6. What happens when Fehling A get mixed with Fehling B solution?
When Fehling A solution is mixed with Fehling B solution, the complex named bistartarocuprate (II) complex is formed and finally, Fehling solution is formed.
Physical Separation Methods
Introduction
Physical separation methods are such methods in which the chemical and physical attributes of elements of any mixture remain the same after separation. Almost all separating methods are useful and widely applied in the current times for different industrial and home purposes. The physical separation method is a supportive and useful method of segregation.
What is physical separation?
Physical separation is associated with differentiating some components based on their structure, gravity, magnetic attributes and some other natures. The optical properties and electrical attributes are also important considerations that are required at the time of separating components in a physical manner. Handpicking, winnowing, threshing, distillation, magnetic separation and evaporation are mostly recognised methods of separating elements from any mixture.
Physical Separation Methods
Some most important and considered physical separation methods are as follows.
Handpicking
The handpicking separation method is one of the simplest methods of physical separation. This method is applied at the time of picking a very small amount of unwanted materials from any grains and large particles. The separated particles may lack purity and therefore can create difficulties in getting a pure mixture. An example of applying handpicking separation method is separating rotten food grains from a sack of wheat grains.
Threshing
At the time of harvesting any particular crop, the threshing method is applied. The harvesting procedure of wheat is performed and followed by the threshing separation method.
Winnowing
Grains are collected from the field and after undertaking the threshing process; all unwanted husks are to be separated. At this stage, the winnowing physical separation method is helpful.
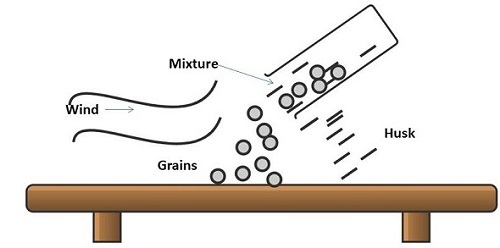
Figure 1: Winnowing method
This separation is performed in an area where the presence of wind is ensured. The grains are thrown from a distance and the winds help in separating all the chaff and husks from grain particles.
Sieving
In some mixtures, components are found to be of different sizes. A sieve is used in separating the larger particles from the smaller ones. At the time of sieving, the smaller particles are passed through the sieve and the large ones remain in the sieve.
Distillation
In a liquid mixture distillation, method is used in separating different types of liquids.
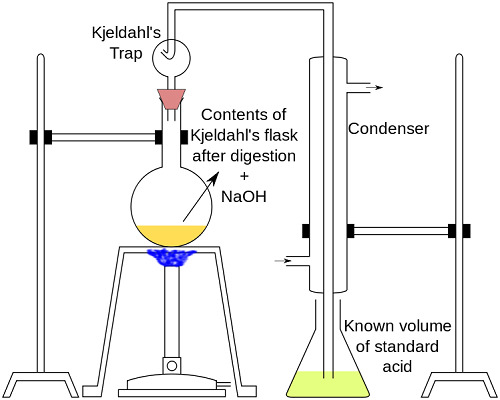
Figure 2: Distillation method
In this separating method, components of the mixture are vaporized and isolated. The evaporation is connected to this. The generated vapour is collected in the jar in a liquid state.
Sedimentation
The sedimentation method of separation is applied in separating a liquid from insoluble solids. As an example, muddy water can be left to rest and after some time, the mud is sediment at the bottom of the jar.
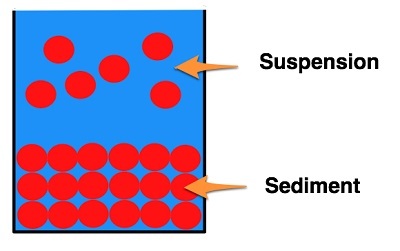
Figure 3: Sedimentation process
All heavier impurities are found to be separated by this method.
Evaporation
A soluble solid and solvent are separated by undertaking the evaporation separation method. All the organic solvents are turned into gas and this process leaves the solid residue left in the jar.
Magnetic Separation
In any mixture, some of the components may be of magnetic properties. A strong magnet is used in separating all magnetic elements such as cobalt, iron, and nickel from the mixture. Metals for any discarded waste can also be separated by the magnetic separation method.
Separating Funnel
The usage of a separating funnel is found mainly for separating more than one immiscible liquid. The nature of the liquids’ density is unequal and this advantage is granted for separating any particular liquid from the mixture. As an example, a mixture of oil and water can be separated by using a separating funnel.
Importance of Physical Separation Methods
All separating methods possess an individual significance in relation to their application to various fields.
In the technological field, new challenges technology can be faced with efficiency by taking the help of the most applicable physical separation method.
Necessary primary elements can be separated from secondary elements by following separation methods.
Reduction of waste is one of the most beneficial aspects of using the separation method with which waste materials are separated from unnecessary ones.
Consumption of pure water is a basic need of living beings and using the purification separation method, and distillation method, this necessity can be fulfilled.
Physical Separation Methods: Application
Some of the most important applications of the physical separation method are as follows.
In a mixture where the components possess different sizes, applying filtration and sieving separation method are very important and helpful.
One may follow the distillation separating method at the time when an impure liquid is to be separated from a liquid mixture.
In some significant industrial purposes, separating magnetic elements from a large amount of fluid and hard substances is done by applying the magnetic separating method.
Identification of radioactive wastes is done by following chemistry related new separation method.
Conclusion
Physical separation methods are often undertaken when it is not necessary to change the chemical and core nature of any element. Each physical separation method possesses an individual technique that is needed to be followed properly for separating the desired elements from a mixture. Any unwanted material is also separated from a necessary mixture without changing its core componential attribute. Most of the unwanted or desired materials are separated from a mixture by following appropriate separating methods.
FAQs
Q1. Which types of mixtures are there those elements can be separated by following separation methods?
Ans. The main two types of mixtures are there namely, homogeneous and heterogeneous. The physical characteristics of these mixture elements are important in this separation method.
Q2. How is the biochemistry field improved by the application of the separation method?
Ans. In the field of biochemistry, the separation of different chemicals is done by applying associated separation methods such as filtration and sedimentation. These two methods are useful for separating biochemistry-related components.
Q3. How is chromatography developed by the application of separated methods?
Ans. An important application of separating methods is found in some theoretical advancement. Chromatography is such an area where the application of a proper separating method is essential.
Physical Properties of Haloalkanes
Introduction
Haloalkane is mainly responsible for the depletion of ozone layer. These particular halogen compounds have several applications for different purposes like in the treatment of malaria and it is used in the time of surgery.
What are Haloalkanes?
Haloalkanes refer to the hydrocarbons that comprise one as well as more atoms of hydrogen that are mainly replaced with the atom of halogen. Haloarenes are another type of hydrocarbon and the main differences between these two hydrocarbons are haloalkanes mainly derived from open-chain hydrocarbons while other hydrocarbons are derived from aromatic hydrocarbons.
Haloalkanes: Physical Properties
The physical properties of this chemical agent are mostly like the other covalent particles. It is very reactive with the functional group. The atomic mass of this chemical compound is mostly different from the other types of chemical elements.

Figure 1: Different melting points of Haloalkanes
Melting Point
This point is generally higher than the other types of alkanes and in the case of the atomic number of carbon is similar in both haloalkanes. Fluoroalkanes are the exception as their melting point is lower than other types of alkynes. For example, this point of methane is -182.5°C while tetrafluoromethane is -183.6°C. This chemical element is mostly colourless as well as the odourless element. The melting points of this chemical compound are different and that is why the melting point is also different.
Boiling Point
Haloalkanes have a higher boiling point than other types of alkanes because of their atom of carbon. For example, 1-Bromo-2-chloroethane takes more heat to boil than chloroethane and it is fully dependent on the atomic weight of alkanehalides. Because of the lower heat consumption, fluoroalkanes take lower heat for boiling. The boiling points of different haloarenes are different and the sequence is Iodoarene > Bromoarene > Chloroarene.
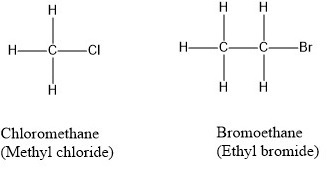
Figure 2: Haloalkanes
Density
The density of this chemical element is generally higher and it is directly proportion” with the compound mass and that is why the density gradually decreases in the lower series of homologous. The density of this chemical element is increased because of the increment of the mass. The density of fluoro derivatives is lower than the chloro-derivatives. Hence, chloro-derivatives are less dense compared to bromo-derivatives.
Solubility
This chemical element is hardly soluble in water because it has a larger amount of relative energy as per requirement. The relative energy is needed for breaking down the chemical bond between two different particles carbon and halogen. A little amount of energy is released during the formation of this bond. It mainly happens after the dissolution of different ions as well as water.
Reactivity
The power of reactivity of this particular chemical element is dependent on the adjacent alkenes. The reactive power mainly increases with the increment of atomic mass weight of the halogens. The nature of Haloalkanes is polarized and that is why it acts like a solvent. Many chemical reactions prove that this chemical element has better power of solvents compared to alkenes.
Haloalkanes: Chemical properties
The chemical features of this chemical agent are as follows −
Carbon is attached to the halogen and it is deficient in electrons. That is why haloalkanes have power that is more reactive the nucleophiles. The reaction of tert-butyl bromide and hydroxide can be presented as
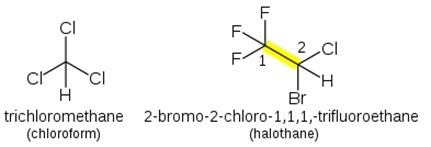
Figure 3: Organic bond of Haloalkanes
Fvasconcellos 20:09, 8 January 2008 (UTC). Original image by DrBob contribs)., IUPAC-haloalkane, CC BY-SA 3.0
Haloalkanes display different types of radical reactions that are free and the Grignard formation mainly takes place with the help of haloalkanes and magnesium (Mg). The mechanism of this reaction can be denoted as −
that is transferred to
After that, the equation changes to −
and finally the reaction changes to −
The formula, R refers to the group of alkyl, X stands for halogen and RMgX denotes the reagent of Grignard.
The substitution reaction is generally seen in this chemical element and the reaction is mainly presented as −
These chemicals mainly undergo the reaction of reactions and the chemical reaction mainly denoted as
Applications of Haloalkanes
The applications of this chemical element are seen in different purposes and in different sectors.
It mostly applies like a retardant of the frame.
This chemical element is also used as fire extinguishers well as refrigerants.
Another application of this chemical particle is in propellants as well as solvents.
In the field of pharmaceuticals, it is mostly used. It is mainly used as the non-polar compounds”
Conclusion
The physical properties of a particular chemical compound are such properties that are closely related to the physical aspects of any compound. The size, shape colour, as well as mass, are included in this type of property. The nature of this chemical agent is hydrophobic. It is flammable but not more than alkanes because the CH bond of this particle is fewer than alkanes.
FAQs
Q1. What are the different types of boiling points of haloalkanes?
Ans. Methylpropane boils at the temperature of -11.7°C while 2-fluoropropane requires -10°C. Because of the increment of this heat consumption, the “boiling point” of isomeric reduces. For example, 1-bromobutane boils at the temperature of 375K whereas 2-bromopropane boils at the temperature of 346K.
Q2. What is the different density value of haloalkanes?
Ans. There are several density values of different types of haloalkanes such as the density value of is 1.336 g/mL and the density value of is 1.335 g/mL. The density of is 1.595 g/mL.
Q3. What are haloarenes?
Ans. Haloarenes are mainly formed with the replacement of atoms of hydrogen in terms of an Aromatic hydrocarbon. Examples of haloarenes are Chlorine, Iodine, and Bromin.
Physical Properties of Amines
Introduction
Amines are chemical compounds that display a distinct set of physical properties that are associated with the connectivity with the carbon atom. This product is quite similar to that of ammonia in terms of structure. There are four different kinds of amines that are formed depending on the replacement of hydrogen atoms by ammonia molecules.
What are Amines?
Amines are chemical compounds that have a distinct set of properties associated with them. Amines are molecules as well as functional groups that are found in organic chemistry, the compound possesses a lone pair of basic nitrogen atoms. Amines are officially considered to be derivatives of ammonia that have one or multiple hydrogen atoms replaced by alkyl or the aryl group. Some of the important amines are amino acids, biogenic amines, trimethylamine and aniline. Mainly, there are three different kinds of amines to be found in nature, which are classified as primary, secondary, and tertiary amines. The classifications are conducted based on the replaced amount of the hydrogen atoms from the ammonia.
Figure 1: Amine
Classification of Amines
Primarily, classifications of amines are conducted based on the replaced number of hydrogen atoms from the ammonia. The different types of amines are discussed below −
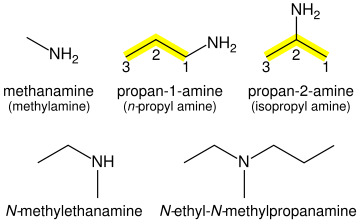
Figure 2: Classifications of amines
Fvasconcellos 20:17, 8 January 2008 (UTC). Original image by DrBob contribs)., IUPAC-amine, CC BY-SA 3.0
Primary Amine
In case one hydrogen atom of ammonia molecule is substituted by alkyl or the aryl group it is known as primary amine. Since this kind of amine retains only one substituent on the nitrogen, these are named along with the substituent as a prefix. It is also called 1° amines. This kind of amine is produced when salt is made in the first stage that is acknowledged as ammonium bromide.
It is similar to ammonium bromide however; an ethyl group substitutes a hydrogen atom that is found in the ammonium ion. Ammonia gives out hydrogen atoms from ethyl ammonium ion, producing primary amine.
Secondary Amine
Two different organic substitutes are utilised to get rid of excess hydrogen atoms in the ammonia molecule that produces an amine. The ammonia gives off the hydrogen ion from dimethyl ammonium ion that leaves secondary amine that is also termed diethylamine.
Tertiary Amines
This kind of amine is produced when a particular organic compound substitutes all the hydrogen atoms. It can either belong to an aryl group or it might belong to an aromatic group. This is prepared, as ammonia removes hydrogen ions from triethylammonium ion that leaves a tertiary amine which is termed triethylamine. Tertiary amine has three alkyl groups bound to nitrogen.
Cyclic Amines
There is another kind of amine while the nitrogen is incorporated into a structure of a ring. It thereby produces either secondary or tertiary amines.
Structure of Amines
Based on the composition of amine, every one of the hybridised orbitals of nitrogen overlaps with the orbits of hydrogen or the carbons. Every amine possesses an unshared pair of electrons in the fourth orbit of nitrogen.

Figure 3: Structure of amine
Kes47 (?), Primary-amine-2D-general, marked as public domain, more details on Wikimedia Commons
The angle formed by C-N-E (where E represents either Carbon or Hydrogen) is less than 109.5° because of the presence of unshared pairs of electrons. The angle of deviation gets a bit lower in cases of trimethylamine which is 108°
Physical properties of Amines
Some of the properties of amines are mentioned below −
Lower aliphatic amines exist in a gaseous state.
Primary amines contain multiple carbon atoms which are liquid at room temperature.
Lower aliphatic amines can form hydrogen bonds with water molecules, it makes the amines soluble in water.
Enhancing the size of hydrophobic alkyl rises the molar mass of amines
The intermolecular association is quite prominent in primary amines as compared to secondary amines. Intermolecular association is not present in tertiary amines.
The boiling point is highest in primary amines and lowest in tertiary amines.
Uses of Amines
Some of the uses of amines are mentioned below −
Amines are used in the production of azo-dyes
They are extensively used in the production of chemicals that protection of crops and food products.
Amines are also helpful in the production of pharmaceutical products. Morphine and Demerol are helpful as painkillers.
Amines are helpful as pest control agents.
Amines help in the tanning of leather
Methamphetamines and amphetamines are considered recreational drugs.
These are also found in personal care products.
Amines are widely found in the global market in the form of ethanol amine
Conclusion
Amines are quite a common substance that is widely used for various functions. The amines are made from ammonia products by substitution of hydrogen atoms with other organic compounds. Primarily, there are three distinct types of amines to be found which have their distinct set of properties associated with them. Amines are mostly found in narcotic and pharmaceutical products. Its associated property of being soluble in water along with other aspects makes it highly useful in different industries.
FAQs
Q1. Is amine flammable?
Ans. In general, amines have a high boiling point, and primary amines have the highest boiling point. These products are combustible but are not extremely flammable in a room temperature.
Q2. What are the chemical properties of amine?
Ans. Amines are organic products having one of multiple nitrogen atoms, structurally theta is often dependent on them. Amines also mirror the structure of ammonia but amines also retain extra properties that are dependent on the carbon atoms.
Q3. Where are amines used?
Ans. Amines is mostly used in the narcotics and medication industry. Different kinds of drugs are produced with the help of this compound. Amines are also found in the production of chemicals for the purification of water. These products are also found in cosmetic and care products. Amines are also found in safety chemicals used for the protection of chemicals.
Physical Properties of Alkynes
Introduction
Alkynes are the most basic organic molecules that are made of carbon and hydrogen. Hydrocarbon is a thing that can be found in nature and that is too in anything that people usually use on daily basis. That is the reason they are considered the parent organic compounds. Any compound can be made by the replacement of hydrogen atom.
What are alkynes?
Alkynes can be defined as non-polar, unsaturated hydrocarbons that have the triple connection between the carbon and carbon particles, which can be represented as -C=C-. The general chemical formula of it is , where the value of n is changed; it can be 2, 3, 4, 5, and many more.
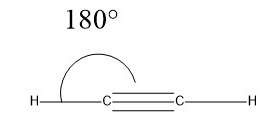
Figure 1: The alkyne: Ethyne
The simplest form of alkynes had at least one triple bond. Alkynes are also known as acetylenes. However, the name sometimes refers to only one kind of alkynes, which is C2H2. The alkynes are also referred to as acetyls and also hydrophobic.
Structure of Alkynes
The bond of alkynes means the H–C≡C bond angles forms 180° angles and because of the angle, they look like a rod. The triple bond of it is so strong that the “bond strength is 839 kJ/mol. However, the bonding is discussed based on the molecular orbital theory, in which the triple bond is recognized as arising from the overlap of s and p orbitals.
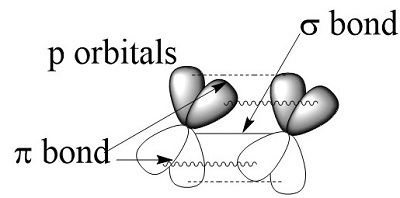
Figure 2: Structure of Alkynes
As the number n in the changes, as per the IUPAC framework, the names of each alkyne form in a specific way. These all have a postfix -yne and the prefix depends on carbon particle numbers. For example, the ethyne’s carbon atom number is 2 and the formula is , the same thing happens with the “Butyne, where the carbon atom number is 4 and the formula is .
Some other alkynes are- Propyne, Pentyne, Hexyne, Heptyne, Octyne, and their carbon atom number are 3, 5, 6, 7, 8 respectively.
Isomerism in Alkynes
The structural isomerism of alkynes is mainly of four types and they are −
Chain Isomerism: In this kind of isomerism occurs when the alkynes have five or more carbon atoms that owe to various carbon chain configurations.
Position Isomerism: In this kind of isomerism, the isomers differ based on the triple bond location.
Functional isomerism: In this isomerism, the functional isomers of the dienes the compounds contain two of the double bonds.
Ring chain isomerism: In this kind of Isomerism, those show ring chain isomerism with the cycloalkanes.
Physical Properties of Alkynes
The most common physical properties of the alkynes are somewhat similar to the alkanes and alkenes −
The physical State of Alkynes
They are the unsaturated carbon that has triple security at the carbon site. The first three members of the group are colorless and present in the gas form while the remaining eight members are liquid and other than that, higher alkynes are solid in the form. The alkynes are the slightest polar in nature.
Smell or Odour
All the members of alkynes are odourless but the acetylene has a garlic-like smell. The reason behind it is the presence of impurity phosphine. Other than that, the ethylene has the slightest odour.
Boiling and Melting points of Alkynes
The melting and the boiling point of the alkynes depend on the molecular mass. Both points increase with the rise of the molecular mass. If the melting and boiling point of the alkynes are compared with the alkanes and alkenes, these are higher.
Solubility
The alkynes show a soluble nature in the case of non-polar solvent and the case of polar solvents are insoluble, such as water.
Density
The density of the alkynes increases with the increase of the molecular weight. If they can be compared with water, they are light. The density range of the alkynes is 0.69–0.77 g/cm3.
Uses of Alkyne
The alkynes are used in many sectors, such as −
The ethyne has exceptionally fire that is the reason they are used in the oxyacetylene gas for welding and cutting.
They are used as fuel.
It is used to make natural mixtures such as ethanol, ethanoic corrosive, and acrylic corrosive.
They are used as gas lights.
Acetylene is used for making the fruits ripen.
They are used to make cis and trans alkenes.
Conclusion
The alkyne has a unique structure that makes it different from the other compounds and essential in the field of organic chemistry. They are majorly two types, that are terminal and internal alkynes. As with any other hydrocarbon, the alkynes are also hydrophobic by nature.
FAQs
Q1. What are the main properties of alkynes?
Ans. The alkynes are the unsaturated hydrocarbons and their physical properties are similar to the same of alkanes and alkenes. The most important of them are -dissolve in organic solvents, and have the slightly dissolvable in the polar solvents. However, they do not get dissolved in the water.
Q2. Do alkynes have acidic nature?
Ans. Alkynes are by nature acidic and the reason behind it is that they can “release the hydrogen atoms and then can form the alkyne ions. Because of the acidic nature of alkynes, they can be used in the form of Brönsted-Lowry acid”.
Q3. What is the main use of the alkynes?
Ans. Alkynes are used as the lanterns that help in the cave exploration. It is also used in the oxyacetylene torches as fuel. Because of its capability of producing high temperature, it is preferred in the welding and cutting metals.
Q4. Why do alkynes have a higher boiling point?
Ans. The boiling point of the alkynes rises with the increase of molecular mass. Not only that, but the melting point also rises as an effect of that.
Physical Properties of Alkanes and Their Variations
Introduction
Alkanes are considered important as these are crucial raw materials predominantly necessary in the chemical industry. More to this, alkanes are used as a primary ingredient in the gasoline as well as in lubricating oils. One of the most crucial properties of alkanes is combustion that states a condition when in the presence of oxygen, alkane’s burn forming the resultant products carbon dioxide as well as water.
What are Alkanes?
Alkanes are known as paraffin and these are organic compounds that consist of both the atoms of carbon and hydrogen. Covalent bond is noted among alkanes that display a structure that is tree-like.
Figure 1: Structure of Alkanes
Fvasconcellos 20:06, 8 January 2008 (UTC). Original image by DrBob contribs)., IUPAC-alkane-5, CC BY-SA 3.0
The formula that is followed by alkanes is expressed as, respectively, and possesses both physical and chemical properties specific to the numbers of bonds present with a number of atoms of carbon and hydrogen. The most popular structure of alkane is methane that is . Alkanes are saturated hydrocarbons having sigma bonds.
Physical properties of Alkanes
Physical properties are the characteristic features of a substance are easily measured as well as observed, without any changes in the identity of the substances. For alkanes, physical properties involve its respective structure, nature of solubility, and boiling and melting points stated as follows −
Structure
In the compound of alkanes, the atoms of carbon present display the form of hybridised. This has four sigma bonds that occur between the carbon and hydrogen atoms.
Figure 2: Electronic Structure of Alkanes
1840460mahesh, Alkanes structers 3, CC BY-SA 4.0
The structure of methane is tetrahedral and symmetric in nature. The bond angles are 109.47°.
Solubility
The solubility of alkanes depends on the bonding of the atoms of hydrogen and carbon. There lays a little difference thereby resulting in the occurrences of electronegativity and they have a bond that is covalent in nature. The alkanes are non-polar in nature and suggest solubility in non-polar solvents. Alkanes are said to be hydrophobic and are not soluble in water.
Boiling Point
The boiling point of alkanes is stated by the Van Der Waals intermolecular forces. This force tends to increases with the increase in the size of molecules, or with the “increase” in the surface area.
Figure 3: Boiling Point
AgnesLager, Alkane boilingpoints (centigrade) from methane to nonadecane, CC BY-SA 4.0
The boiling point of alkanes is stated to increase depending on the increased weight of the weight of the molecule
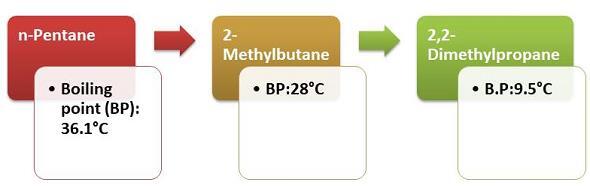
Figure 4: Exceptions to Boiling Point of Alkanes
The alkanes have a straight chain which results in the higher value of boiling point. This is, however, in comparison to the structural isomers noticed for alkanes.
Melting Point
The melting point is quite similar to the boiling point, noticed among the compounds of alkanes. Therefore, the melting point increases with an increase in the molecular weight of alkanes.
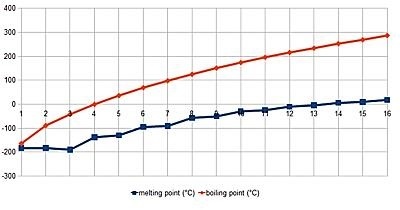
Figure 5: Melting Point
1840460mahesh, Alkanes boiling and melting point graph, CC BY-SA 4.0
The higher alkane is noticed to be solids and therefore it is quite difficult to break down intermolecular forces.
Other properties of Alkanes
Other properties of alkanes include chemical properties. The chemical properties involve two major characteristic features of alkanes are combustion and halogenation.
Combustion
The process of combustion is referred to as a chemical reaction that occurs between oxygen and substance. However, this reaction takes place when heat and light are given to it. The group of alkanes are high in combustion and instantly conducts the process in the presence of sufficient oxygen. The reaction is expressed as,
However, an exothermic reaction is noticed in the process of combustion of the alkanes. Therefore, alkanes are used in the production of fuels.
Halogenation
The reaction of Halogenation results in the formation of derivatives of hydrocarbons. This results in the substitution of one or more atoms of halogen from the atoms of hydrogen. The reaction of “Halogenation reaction is expressed as −
Importance and Application of Alkanes
In the aspect of industries, alkanes are considered to have high commercial usage. The application of alkane includes, as a major component of LPG or liquefied petroleum gas, and making of petroleum jelly. More to this, the alkanes are used in jet fuels and due to the high viscosity the alkanes are used as lubrication oils, in making candles and making of synthetic polymers.
Conclusion
In this tutorial, the focus has been given to comprehending the properties of alkane compounds. This not only includes physical properties as well as chemical properties. The structure and the alkanes are discussed that show the tetrahedral structure and it has sigma bonds among its carbon and hydrogen atoms.
FAQs
Q1. What is the properties noticed for alkanes?
Ans. Alkanes are known to be the simplest in the family of hydrocarbons. These compounds have carbon and hydrogen that consist of bonds that include carbon-hydrogen and carbon-carbon. These compounds are seen to be quite less in their reactivity level together with very less biological activities. More to this, alkanes are unique as they have no colour in them and they are as well odourless.
Q2. What is considered as the physical state for higher alkanes?
Ans. Higher alkanes are most times used as literal sense at times that simply means alkanes have a higher number of atoms of carbons. Another definition states that higher alkanes are considered as n-alkanes. These n-alkanes are seen to be solids under natural conditions.
Q3. What are the varied types of alkanes found?
Ans. In the field of chemistry, majorly three kinds of alkanes are found that involve, the straight chain of alkanes, the branched chain of alkanes and lastly, cycloalkanes.
Physical Properties of Aldehydes and Ketones
Introduction
Physical properties of aldehydes and ketones possesses such as the boiling point, melting point, normal boiling-point range, refractive index, density or specific gravity or solubility parameter can all be manipulated to determine an aldehyde or ketone. The components of the physical properties are useful in chemistry to determine several backgrounds. The electronegativity which is a component of the physical properties can make differentiate two elements like Aldehyde and Ketones.
Information about Aldehyde and Ketones
Aldehydes and ketones are combinations that prevent a carbonyl group, and therefore, these combinations are collectively cried carbonyl combinations. There is a dual bond between the substances of oxygen and carbon. Due to the distinction between the dual bonds complexity takes place. The carbonyl bond is more reactive to nature as it contains polarity.
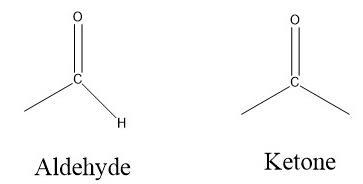
Figure 1: Aldehyde and Ketones
Aldehydes
The boiling point of an aldehyde is more elevated than the connected alcohol because of the electron-withdrawing influence of the carbonyl group. The boiling point of an aldehyde advances with the advancing length of the carbon chain. The melting point of an aldehyde is negligibly more increased than the boiling point due to the molecules is held together by hydrogen bonds.
Ketones
The boiling point of a ketone is more elevated than the boiling point of alcohol because of the electron-withdrawing influence of the carbonyl group. The boiling point of a ketone enhances with the length of the carbon chain.
The melting point of a ketone is also more than the boiling point due to the presence of the hydrogen bonds that held the molecules together. The standard boiling-point range is negligibly lower for ketones than aldehydes. This is due to the ketones are less polar than aldehydes. Consequently, ketones have a more fragile exchange with the molecules of water.
Physical Properties of Aldehydes and Ketones
The followings are the physical properties of Aldehydes and Ketones −
Physical State
Methanal is a gas that can make a strong odour. Ethanol is a flammable fluid. Various aldehydes and ketones persisting up to eleven carbon atoms that are colourless fluids while still more increased components are solids.
Smell
Except for the aldehydes of the low carbon, which contain odours which are not pleasant for anyone, other aldehydes and ketones contains odour which is considerable and pleasant. With the size of the molecules, the odour evolves less aromatic and better perfumed. In reality, many unpretentiously emerging aldehydes and ketones have been utilised in the blending of perfumes and flavouring agents.
Solubility
The four atoms of carbon in the Aldehydes and ketones are miscible in the water. Due to the presence of the hydrogen bond associated with the polar carbonyl group and water molecules as stated in the following section −
Nevertheless, the solubility of aldehydes and ketones in water declines rapidly on supplementing the length of the alkyl chain. As a consequence, the higher components with more than four carbon atoms virtually showcase the nature of insoluble in water. All aldehydes and ketones are soluble in organic solvents such as benzene, ether, chloroform, and alcohol.
Boiling Point
The boiling points of aldehydes and ketones are more elevated than non-polar compounds due to the weak polar combinations of identical molecular masses. In addition to this, the boiling points are lower than alcohols or carboxylic acids due to the aldehydes and ketones being polar combinations controlling adequate intermolecular dipole-dipole interactions among the contrasting endings of C=O dipoles.
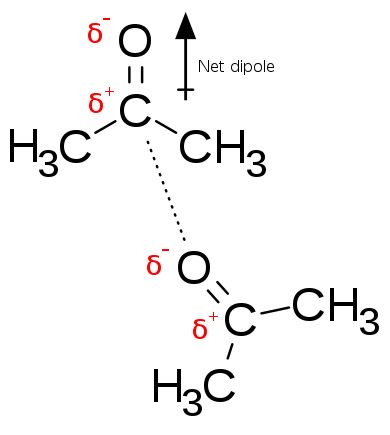
Figure 2: Dipole-Dipole interactions
User:Innerstream, Acetone dipole-dipole, marked as public domain, more details on Wikimedia Commons
Accordingly, the dipole-dipole interactions are not strengthened for the intermolecular bonding of the hydrogen adhesion in alcohols and carboxylic acids. Additionally, the boiling points of aldehydes and ketones are low than the alcohols and carboxylic acids of the molecular masses.
Other vital properties of Aldehydes and Ketones
The chemical properties of Aldehydes and Ketones are described in the following section −
Reaction with Alcohols
The reaction between Aldehydes and alcohols in the existence of dry HCl gas can possibly deliver gem-dialkoxy compounds. These combinations are known as acetals.
Reaction with Hydrogen Cyanide
An additional product named cyanohydrins formed due to the reaction with the hydrogen cyanide with aldehydes and ketones. The reaction can experiment in the presence of aluminium chloride.
Facts about Aldehydes and Ketones
The construction of iodoform is operated as a test for certain aldehydes and ketones. Consequently, it has methyl groups bonded to a carbonyl group. This examination is maintained in the presence of sodium carbonate and iodine solution. This response is known as the iodoform examination.
Conclusion
The availability of the polar carbonyl groups can make a justified boiling point that can increase the molecule size where the additional factors are reliable to conduct gem-dialkoxy. In aldehydes, the carbonyl group is connected to one hydrogen atom and one alkyl or aryl group, however, it's not the same in ketones, it is connected to both alkyl and aryl groups. Both physical and chemical properties are vital to examining the significance of chemistry.
FAQs
Q1. Which examination is utilised to recognise Aldehydes and Ketones?
Ans. The iodoform examination is employed for the purpose of Aldehydes and Ketones which is one of the adequate reasons for testing the sodium carbonate and iodine solution. The presence of the Aldehydes and Ketones can be recognised easily with the iodoform test.
Q2. What is the shape of the carbonyl molecule?
Ans. The carbonyl molecule’s shape is trigonal planar. The formation of the sp2 hybridized can make three bonding of the orbital and nonbonding orbital.
Q3. What will happen if Sodium Bisulphite reacts with Aldehydes and Ketones?
Ans. Both the elements aldehydes and ketones form crystalline expansion combinations called bisulfite adducts when it is treated with a saturated solution of sodium bisulphite.
Physical Properties of Alkenes
Introduction
Alkenes are determined as clear or colourless, double-bonded substances. Ethane that is denoted as alkenes is the colourless gas having a sweet odour that can intoxicate or make a person faint. The first 3 elements that are present in groups of alkenes are gases, the next 14 elements are in a liquefied state and the rest of the compounds of alkenes are in a solidified state.
What are Alkenes?
Alkenes are determined as the vital hydrocarbons having the formula . Alkenes are made up of the C=C functional group. Alkenes compounds are determined as unsaturated that generally undergo various additional reactions. Alkenes can also be utilised in the synthesis of alcohols, plastics, lacquers, detergents, and fuels as primary materials.
Types of alkenes
Some major types of alkenes that are widely used in manufacturing industries are discussed.

Figure 1: Types of alkenes
Propene
Propene can also be referred to as propylene used to produce apparel, plastic squeeze bottles, outdoor furniture as it has the properties of plastic resin. Propylene oxide can also be utilized in order to manufacture furniture, automotive parts, boats and recreational vehicles, appliances, solvents and resins.
Butene
Butene is utilized for the formation of various chemicals present in gasoline and rubber processing factories. Butenes components are widely used as alkylate and polymer gasoline. The compost of Butene often helps in the production and conversion as fuel, including fuel gas or blendstock in the case of gasoline.
Pentene
The major compound present in the alkene group is the n-Pentene which is a clear liquid. Pentene has a very disturbing and unpleasant smell. The compound is widely used as pesticides, additive to gasoline, and in the production of several vital chemicals.
Physical properties of Alkenes
Some common physical properties of alkenes are mentioned below −
Melting Point
Melting point of alkenes compounds that are generally denoted as double-bonded compounds highly depends on molecular positions. Alkenes have similarities to the melting points of alkanes. In this case, the cis-isomer molecules have a lower melting point whereas trans-isomers molecules remain packed within a shape that is denoted as the U-bending shape.
Solubility
Solubility of alkenes in water is poor because of their non-polar characteristic features. The non-polar solvent in which the alkenes show proper solubility includes benzene and ligroin.
Boiling Point
Bolimg points of alkenes are enhanced with the increase of atoms of carbon in the compounds. Boling pints are very similar to the compounds of alkenes and alkanes. Compounds of alkenes and alkanes consist of similar carbon skeletons or structure. The straight-chain alkenes compounds have a greater boiling point than branched-chain alkenes.
Polarity
Like alkanes, alkenes are also determined as weakly polar compounds. Alkenes are more reactive than alkanes as they have double bonds. The π electrons usually forms a double bond that can be removed easily or can be added as they are joined weakly. The “dipole moments that are generally exhibited through alkenes are greater than alkanes. Alkenes polarity highly depends on the functions group that remains attached to the chemical structure and the compounds present in Alkenes groups.
Chemical properties of Alkenes
Alkenes have the three major kinds of primary reactions that are generally denoted as the addition reaction as stated below.
Addition of Halides
Alkenes react with halides through a specialized rule determined as the Markovnikov rule. According to this rule, it can be stated that the portion of reactant’s negative remains attached to a carbon atom and the least number of atoms of hydrogen. This reactant’s negative part is generally denoted as the molecule that can be added to a chain.
Addition of Halogens
In the reactions of halogens and alkene, the production of vicinal dihalides is seen. Iodine does not react with alkenes like other forms of halogens. On the other side, bromide reacts with any of the alkenes and remains attached to the site that is unsaturated in nature. The proof of the unsaturation state can be seen in the reaction −
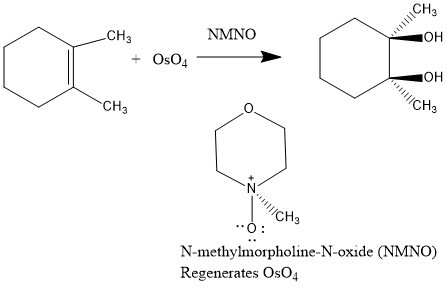
Figure 2: Dihydroxylation of alkenes with Osmium Tetraoxide
Addition of Hydrogen
Alkenes react with the molecular chain of one diatomic molecule of hydrogen that can also be determined as a dihydrogen molecular chain in presence of nickel and platinum. The molecules of nickel and platinum become alkenes because of the specialized rearrangement of atoms.
Uses of Alkenes
Several uses of compounds of alkenes are stated below −
Alkenes compounds are utilized in the manufacturing processes of plastics and polythene materials including buckets, bowls, bags, and many more.
The compounds of alkenes are used in the production of anti-knock for automobile engines like cars’ engines.
In manufacturing industries, alkenes compounds like “plastic and polypropene” are often used in order to make ropes and packaging material.
Major compounds of alkenes like ethane-1,2-diol are utilized in anti-freezing for motor car radiators.
Conclusion
Alkenes can also be used in “ethanol and synthetic fibre terylene” production. The “acrylic fibres and propanol” are included in the group of alkenes that helps in the production of acetone. In the reactions of hydrogen halide and alkene, the hydrogen remains attached to other hydrogen atoms. The bond that is formed here is the double bond. On the others side, there is a halide ion that will be attached to a carbon atom that has a few atoms of hydrogen attached to it.
FAQs
Q1. What are the properties of propylene?
Ans. Propylene is generally referred to s propene which is the clear gas having a melting point of −185.2°C (−301.4 °F; 88.0 K) and a boiling point of −47.6°C (−53.7 °F; 225.6 K). The density of propylene is 1.81 kg/m3, gas (1.013 bar, 15 °C).
Q2. What are the major types of alkenes widely used in manufacturing industries?
Ans. Ethane-1,2-diol, plastic and polypropene are generally used in the manufacturing industry. The components are widely used in the production of automotive parts, boats and recreational vehicles, and furniture.
Q3. What are the major types of alkenes used in manufacturing industries?
Ans. The compounds of alkenes that are highly used in various manufacturing purposes include Hexene , Heptene , and Pentene . Synthetic fibre terylene are highly used in the manufacturing of bags, buckets, and bowls.
Unit Cell Packing Efficiency
Introduction
Unit cell of packing efficiency is referred to the development of the particles of constituents of any specific structure or cell with the presence of void spaces between the atoms. The matter of packing cells is obtained with any specific volume that occupied by the particles that are constituent with the total volume of cells. Packing of the unit cell efficiency is often described with a percentage value.
Information regarding Unit Cell Packing Efficiency
A unit cell is described as a structure having three dimensions and constructed by one or more atoms. Additionally, the 3D object is used to make to view a unit cell. The moment when there is packing in the unit cell, it has been found that a certain void is available in it.
The stretch is loaded by other components or particles. The trace of absolute space that is replenished with the special cell or structure is named the packing fraction. In Chemistry a percentage view is essential for the development of knowledge regarding the effectiveness of the particles that are constituents. The notion of the structure delivered effective knowledge regarding the unit cell packing in chemistry.
A lattice referred to a largely constructed number of unit cells in which the point of the lattice is either filled or occupied by a constituent particle. The point of lattice for a unit cell is a concept that seeks a structure of three-dimensional that is referred by a 3D structure also.
The cubic closed or packed or the cap and the hexagonal closed packed or hcp are two efficient lattices with the consideration of packing in chemistry. Both of the packing refer to the 74% of the space is filled. For instance, a cubic lattice has a packing efficiency of 52.4% and 68% for the body-centred cubic lattice or bcc.
Packing Efficiency of the Body–Centred Cubic (BCC) Unit Cell
BCC is the kind of atomic arrangement array refers to the particles available on eight edges of a box structure and a single particle at the centre of body or box. The following formula is taken into consideration for the efficiency with the process of calculation based on the following expression −
Additionally, the above-furnished equation keeps two atoms and area occupied by one atom is . The efficiency of BCC Packing is considered as 68.04% and rest of 32% of the volume remains void.
Packing Efficiency of Simple Cubic Unit Cell

Figure 1: Packing Efficiency in Body-centred Cubic Unit Cell
Thus, packing efficiency = volume occupied by one atom total volume of unit cell x 100.
Hence, the packing efficiency of the simple cubic cell (SCC) unit is 52.4%.
Packing Efficiency of Hexagonal Close Packing (HCP) and Cubic Close Packing (CCP)
Both the HCP and CCP are referred to the structure that has identical packing efficiency with the relation between both sides is presented as the radius are presented as r.
Figure 2: Efficiency of FCC, BCC, and simple packing of atoms
Cubique_centre_atomes_par_maille.svg: Cdang (original idea and SVG execution), Samuel Dupré (3D modelling with SolidWorks) derivative work: Daniele Pugliesi (talk), CCC crystal cell (opaque), CC BY-SA 3.0
Cdang, Cubique a faces centrees atomes par maille, CC BY-SA 3.0
CCC_crystal_cell_(opaque).svg: *Cubique_centre_atomes_par_maille.svg: Cdang (original idea and SVG execution), Samuel Dupré (3D modelling with SolidWorks) derivative work: Daniele Pugliesi (talk) derivative work: Daniele Pugliesi (talk), Simple cubic crystal cell (opaque), CC BY-SA 3.0
The following equation referred to the use case of both packing technique in solutions −
These blocks referred to as the face-centred lattice point of the cubic and it contains some extra atoms where these are situated on eight corners of the cube and the centre of each wall as well. The volume represents with r and the equation can be stated as the following matter −
The HCP and CCP are referred to as 26% of the void space in the efficiency of packing.
Conclusion
The unit cell is referred to the smallest collection of molecules or atoms forms a crystal when these are referred to as regularly intervals in 3D. The proportion of the total void area to the occupied position by atoms or ions referred to as the packing efficiency in the unit cell. The Hexagonal close packaging is referred to the cubic structures developed by organising and focusing on the body.
FAQs
Q1. What is packing efficiency?
Ans. The percentage of the overall space, which is occupied by the particles in a certain packing, is comprehended as packing efficiency. Atoms, ions, and other particles that constitute are packed closely in the crystal lattice. These can do the process inside the ccp or in the hcp based on the phenomenon. Both of the cases referred to the free space in the number where the void is left, the total space is not occupied.
Q2. What is the packing efficiency in HCP and CCP structure?
Ans. The efficiency of the packing in the CCP and FCC is a cubic structure entered for the face. The atoms in the octahedral void are referred to as the cubic close packing (ccp) is 74%. Additionally, two different names are there in comprehending knowledge of packing efficiency.
Q3. Is CCP and FCC the same?
Ans. The CCP and FCC are the cubic stress that is referred to the development of knowledge regarding the packing of the entered atoms in the cell unit. The void of the packing refers to the ABCABC form that is known as CCP and the unit cell is FCC.
Q4. What is the process of BCC packing in the unit cell?
Ans. The addition of the BCC packing in the chemistry provides knowledge regarding the process of eight atoms in each corner and one atom placed in the centre. The structure of BCC is open in the efficiency of unit cell packing. The equation refers to 8 × 1/8 = 1 atom.
Q5. What is the packing efficiency in SCC?
Ans. The efficiency rate of packing of SSC referred to around 52.4% in the unit cell packing in chemistry.
Difference Between Green Chemistry and Environmental Chemistry
Green chemistry and environmental chemistry are two branches of chemistry that are concerned with the impact of chemical products and processes on the environment. While both fields share some similarities, they have some significant differences. This essay aims to highlight these differences and provide a comprehensive understanding of both green chemistry and environmental chemistry.
What is Green Chemistry?
The field of chemistry known as "green chemistry," or "sustainable chemistry," focuses on developing chemical processes and products that generate as few potentially harmful byproducts as possible.
By adhering to Green Chemistry's tenets, policymakers, institutions, scientists, and engineers may protect and benefit the economy, earth, environment, resources, and people by developing new ways to reduce waste, save energy, and find substitutes for harmful chemicals.
What is Environmental Chemistry?
The study of naturally occurring biochemical processes is known as environmental chemistry. It entails knowing what kinds of naturally occurring chemicals are there, how much of each is there, and what impacts they have in an unpolluted setting. This field of study is essential for determining the extent to which human activities have altered the natural environment through things like the discharge of toxic chemicals from factories and pollution.
Together with chemistry, it incorporates fields like as physics, biology, agriculture, materials science, public health, sanitary engineering, and many more. To sum up, environmental chemistry is the study of the effects of chemical species in the hydrosphere, atmosphere, lithosphere, and biosphere, as well as their origins, reactions, and final resting places; it also investigates their transport and the effect that human activities have on these different parts of the environment.
Differences: Green Chemistry and Environmental Chemistry
The following table highlights the major differences between Green Chemistry and Environmental Chemistry −
Characteristics | Green Chemistry | Environmental Chemistry |
|---|---|---|
Definition | It is an area of chemical engineering that has established and created a set of guidelines, principles, products, and processes that alleviate or eliminate the utilization and generation of hazardous substances at the source. Green Chemistry is a key to sustainable development, as it directs and drives the scientific community to the remedial and innovative solutions for the existing environmental problems. | Environmental chemistry is the branch of science that focusses on the biochemical process occurring in air, water, aquatic and terrestrial establishments and the impacts of pollution and other anthropogenic activities on them. This concept should not be confused with sustainable or green chemistry, which emphasizes to minimize pollution at its source. Environmental chemistry includes topics such as marine chemistry, environmental modelling, biochemistry, geography, astrochemistry, atmospheric chemistry, geochemistry, and pollution remediation. |
Principles |
| Environmental Chemistry does not have any principles but parameters and measurable factors that focus on identification of natural resources, source of pollutants and their impacts. These can include −
|
Benefits | The environmental and societal benefits of green chemistry include
| Human Health
Environment
Economy and business:
|
Conclusion
Green chemistry and environmental chemistry are both important fields of chemistry that are concerned with the impact of chemicals on the environment. While they share some similarities, they have some significant differences, including their focus of research and their focus on the life cycle of chemicals.
Green chemistry focuses on the design and development of environmentally friendly chemicals and processes, while environmental chemistry focuses on the study of the impacts of chemicals on the environment and the development of methods for mitigating these impacts.
Both fields are essential for ensuring a sustainable future, and their integration is necessary for a comprehensive approach to the protection of the environment.
Unsaturated Hydrocarbons
Introduction
Unsaturated hydrocarbons are made up of multiple covalent bonds between one or more than one C-C bonds in a single compound. The field of organic chemistry is usually common to have multiple covalent bonds reside between carbon atoms. In IUPAC nomenclature, the numbering is counted from the side where double bond comes first in counting among the total number of carbon atoms in the chain or ring structure.
Preparation of Unsaturated Hydrocarbons
There proper methods which are able to aid in the production of unsaturated hydrocarbon. The process of preparation is always in need of specific chemical reactions. In these particular processes, carbon monoxide is used to deliver a reaction with the atoms of hydrogen. The reaction is able to occur only in the presence of metals which are able to show the properties of oxides. These metals are very stable in nature for production of the perfect unsaturated hydrocarbons.
A single condition is mandatory for verification of the right way of the reaction from the first step. The condition is like, the metals are used in the reaction must belong to Group II to Group VII in the normal periodic. The metals are taking part in the reaction in its oxide state. There are two important factors, which look after the process of this reaction. The first is the atmospheric pressure, which should be in its rough state. The second factor which influences the preparation of unsaturated hydrocarbons is temperature. In such cases, the temperature must not go beyond 520°C.
Types of Unsaturated Hydrocarbons
Alkene - This is the first type of unsaturated hydrocarbon carries double bond between two C atoms in the organic molecular structure. This type of hydrocarbons makes a single or multiple set of double bonds. The general formula of alkenes is CnH2n signifies a double bond and does not belong to any functional group.
Alkyne - This is the second type of unsaturated hydrocarbon, where, a single set or multiple set of triple bonds are seen to lie between two adjacent carbon atoms. These unsaturated hydrocarbons are not a part of any functional group. Hence, the formula is used to denote this type of unsaturated hydrocarbon is CnH2−n.
Figure 1: Difference between saturated and unsaturated hydrocarbon
Aromatic hydrocarbon - The final type is considered an unsaturated hydrocarbon in spite of being slightly stable. This is the reason behind this type of hydrocarbon has shapes of rings that remain closer to the pi electrons.
Physical Properties of Unsaturated Hydrocarbon
Unsaturated hydrocarbons have two major physical properties like, melting point and boiling point. The other properties include the solubility of unsaturated hydrocarbons and the total number of constituents present in the unsaturated hydrocarbon structure.
Hydrocarbon has a proportion of hydrogen in larger number than carbon. The melting point varies from one another as per unsaturation present in the hydrocarbons like in, alkenes and alkynes. Some of the melting and boiling points are lower than 0°C and some of hydrocarbons exist with above 100°C.
Figure 2: Unsaturated hydrocarbon: Benzene
The second physical property defines the solubility of the element in this context. The unsaturated hydrocarbons are mostly non-polar in nature hence; it is not able to soluble in protic or polar aprotic solvents. However, few of the unsaturated hydrocarbons are able to soluble but in very small in concentrations into polar solvents depend upon its degree of unsaturation. Hence, it is very convenient to mix these unsaturated hydrocarbons into non-polar solvents.
Uses of Unsaturated Hydrocarbons
Many fruits are turned into ripe food items by injecting a small dosage of alkenes.
This type of compound is used in the battlefields because it is used in the production of very poisonous mustard gas.
These are found in the plastic manufacturing units or sectors.
Products like cups, plates, and cartons to store eggs are made up from unsaturated hydrocarbons. The pharmaceutical industries use alkenes to manufacture anaesthetics for surgeries and medical uses.
These are used in the production of alcohol for commercial uses except for human consumption.
Effects of Unsaturated Hydrocarbons on the Human Body
The unsaturated hydrocarbons have severe effects on the human body. These are capable to make a person seriously ill which may cause as far as ending the life of the individual. The hydrocarbons found in the unsaturated state are very poisonous as these compounds produce mustard gas used on battlefields.
These are used in every household. The person who has been exposed to this toxic compound has more chances of getting several heart problems. The organic compound is able to create problems in the kidney and liver when these are used in quite often.
Conclusion
The tutorial explains the three types of unsaturated hydrocarbons with different physical properties. The products are derived using various synthesising processes are toxic to human health. These are produced from metal oxides belong to Group II to Group VII. The synthesis of those elements gives some convenient products used in the daily basis. The images give a brief synopsis of the tutorial to help the learners grasp the context.
FAQs
Q1. What are the preliminary arrangements for the isomers found in an unsaturated hydrocarbon compound?
Ans. The preliminary arrangements for the isomers found in an unsaturated hydrocarbon compound are to develop shapes that are optical, geometrical and isomeric.
Q2. What is a chemical property that helps in the production of hydrocarbons in their unsaturated condition?
Ans. The chemical compounds of unsaturated hydrocarbons are very rich in carbon dioxide and water. This compound starts to get rid of this excess element by going through a process of combustion.
Q3. What is the total number of bonds that is present inside a hydrocarbon compound?
Ans. The total number of bonds that is present inside a hydrocarbon compound starts from one end to the other. In this case, the number of bonds may increase to form a ring of circles around the atom of carbon.
Types of Titration
Introduction
Titration is a quantitative analysis used in chemistry used to identify the strength or concentration of a given unknown chemical solution by analysing it with a known solution concentration with the presence of an indicator.
Both oxidation and reduction happen at the same time. Titration is a process for calculating the quantity of one unknown solution concentration by other solutions. An Indicator is required to detect the endpoint of equivalence. It generally occurs with colour change.
What is Titration?
Chemical reaction occurs with the interchange of ions when certain chemical species are in contact with each other. Titration is a quantitative analysis used in chemistry used to identify the strength or concentration of a given unknown chemical solution by analysing it with a known solution concentration with the presence of an indicator. The known concentration participating in the process of titration is called titrant. Titrated substance indicates the substance whose quantity is to be calculated.
An unknown analyte concentration needs to be added by calibrated titrant concentration” for the identification of the titration endpoint as part of the analysis. The mol count of analyte and titrant will be identical at the endpoint. Titration is helpful for volumetric analysis of different concentrations.
Titration Types
Titration offers a volumetric analysis of solutions. It initiates the estimated volume of solution concentration in an appropriate solvent.

Figure 1: types of titration
The volume estimation process or titration can be classified into four major types. These are: complexometric titration, precipitation titration, redox titration, and acid-base titration. Different titration processes are discussed below.
Titration of Acid and Base
Acid-Base titration occurs between bases and acids, with one being the titrant and other being the titrated. The two concentrations can neutralize each other at the endpoint and produces water and salt. The equation is as follows −
The equivalent point of acid-base titration is indicated by the pH indicator or acid-base indicator.
Titration Procedure
The burette, pipette and conical flask are rinsed with the standard solution, unknown solution and distilled water.
The analyte needs to be with the accurate measurement using a pipette to send to the flask with some indicator drops.
The burette should be filled half by the standardized solution. In addition, the measured volume needs to be recorded.
The estimated amount of known solution is required to keep at this level.
The solution is drained from the burette allowing the indicator colour change at a point and the value needs to be noted.
It is the initial titration, the same procedure is done for three more times, and the average value should be noted.
The difference of initial and final values indicates the total used titrant amount.
The permanent colour change of indicator denotes the reach of the endpoint.
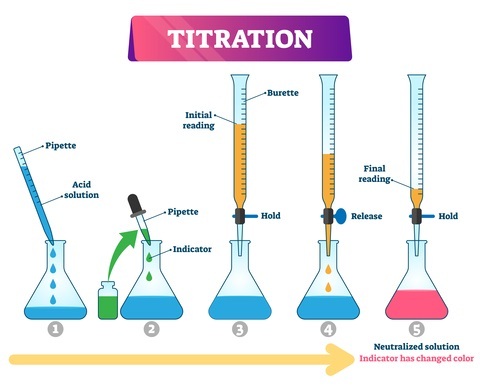
Figure 2: Acid-base titration procedure
The indicators are weak basic or weak acid in nature. The indicator drops are added to the solution one by one for this titration process and neutralization is indicated with a sharp colour change. Methyl orange and Phenolphthalein are two mostly used acid-base indicators.
Redox Titration
This titration is also known as a reaction of reduction and oxidation. Aqueous solution of reacting ions receives electrons in hemical reactions of this titration process. This titration is classified into the following types based on the applied reagents.
Dichromate Titrations
In this reaction, the use of potassium dichromate occurs in acidic medium as an oxidising agent. Dilute sulphuric acid is used for maintaining the acidic nature of the medium. The reaction is −
Potassium dichromate solution is directly used to estimate iodides and ferrous salts.
Permanganate Titrations
In this titration, the oxidizing agent is potassium permanganate. Dilute sulphuric acid is used for its maintenance. The equation is −
The solution has no colour before it reaches the endpoint. Estimation of ferrous salts, acidic acids, oxalates, and hydrogen peroxide can be done with standardized forms of potassium permanganate.
Iodometric and Iodimetric Titrations
In this process, free iodine is reduced to iodide ions, and iodide ions are oxidised afterreleasing electrons. The expressions are −
In iodometric titration, the use of free iodines as indicators as well as an oxidation agent for the reaction and in iodometric titration to generate free iodine.
Complexometric and Precipitation Titrations
Precipitation titration occurs with the contact of two reacting substances with the formation of insoluble precipitate. In sodium chloride or ammonium thiocyanate solutions, the use of silver nitrate solution proceeds with the formation of white precipitate or silver chloride or silver thiocyanate after reactions. The reaction is expressed as −
In complexometric titrations, the formation of an undissociated complex occurs at the point of equivalence. Co-precipitations in this process do not give errors. It is more useful than precipitation titrations method. The expression complexometric titration process is asfollows −
Some metal complexes are formed with the help of a useful reagent is Ethylenediaminetetraacetic acid.
Conclusion
Titration is the quantity measurement process of one substance with the presence of another substance. The substance whose quantity is calculated called titrated and the titrant exists with the known concentrations.
Titration can be divided into different types based on the reaction participant types. These are, acid-base titrations, precipitation titrations, redox titrations, and complexometric titrations. Phenolphthalein, methyl-orange and other indicators are used for identifying the endpoint of the titration process.
FAQs
Q1. What are indicators in titration?
Ans. Indicators are weak acid and weak base substances that make their colour change while identifying chemical changes in solutions. They indicate the change of pH level in a solution that they are participating in.
Q2. Why titration is important?
Ans. Titration helps to understand the concentration quantity of a solution with other acidic solutions and the solutions can be reutilized for accurately determining the concentration level.
Q3. What is the meaning of titration endpoint?
Ans. Titration endpoint is the moment when the required reactant is added in full to a solution in the titration process. It is the terminal point of titration.
Types of Reactions Experiment
Introduction
Different types of reactions experiment may be seen in the particles when these are passing through chemical reactions. Internal as well as external changes can happen in this reaction or experiment. In these types of reactions, the primary substances does not exerts with any changes in physical properties like, the changes of freezing point or evaporation temperature etc.
What is a Reaction Experiment?
Reaction experiment is an integral part of the chemical field and different types of reactions are arranged to form new molecules or change the physical as well as chemical substances.
The excitement of the chemical field is completely dependent on the power of reactivity of the molecules.
Various kinds of experiments are seen and for those reactions, the processes of the experiments are different as well. Combination and decomposition are the most useful and applied experiments in the chemical field. Single displacement reaction experiment along with combustion is other types of reaction are very significant in the chemical field.
Combination Reaction
This reaction requires two different molecules that react and then form a new compound. This kind of reaction is also called a synthesis reaction because in a single reaction, rearrangement along with combination of double or more particles happen for the formation of the product. A simple example of this type of reaction is presented as follows −
Figure 1: Formation of NaCl
The real example of this type of reaction is the formation of NaCl as in this reaction the sodium and chloride are reacted to form sodium chloride. This reaction is presented as
Another experiment of this type of reaction is seen with and in this reaction; a metallic or non-metallic body is reacted with oxygen for forming oxides. This reaction is denoted as −
Decomposition Reaction
This particular type of reaction is a little different from the previous reaction. In this reaction, a complex molecule breaks into a simple molecule. The decomposition experiment requires a specific energy source which is essential for breaking the present bonds of the molecules. It denotes the breaking down of the compounds into a simple compound or molecule. An example of this type of reaction is as follows −
It mainly takes place in the existence of different natural sources like ,light, heat, as well as electricity. A simple decomposition reaction is arranged when a binary molecule is directly composed for forming a new product. An example is as follows −
It displays that the reactant can easily be transformed into a particular form. The form can be element or compound. Through it process, is decomposed with some molecules like calcium oxide along with carbon dioxide. Bases as well as alkalis easily are decomposed after heating. The reaction is denoted as
Single Displacement Reaction Experiment
This is a complex type of compound and in this reaction; a single element of reactant is replaced with an almost similar type of element of various reactants. It is also called a single replacement reaction experiment because it relies upon the chemical reactivity of the molecules. High reactivity elements are mainly replaced with the lower reactive elements in this reaction. It is denoted as −
Figure 2: Reaction of metallic iron displaces copper ion
An example of this experiment is seen between magnesium and copper and in this reaction magnesium mainly replaces copper because the reactivity power of magnesium is higher than copper. It reaction can be presented as −
Another example of this type of reaction is as follows −
Combustion Reaction
This type of reaction is a particular chemical experiment where the reactants are involved in the reaction with the help of oxygen and then release a certain amount of energy as well as heat. This reaction mainly occurs in the existence of oxygen and two different types of reaction can be seen such as incomplete and complete combustion.
It displays that the organic molecules undergo a complete combustion producing and in the gas form and releasing energy. The reaction is presented as −
It shows that the hydrocarbons are applied in this reaction for the production of energy. This type of reaction refers to as an exothermic reaction.
Conclusion
The reactions not only are seen in the laboratory but in the surrounding of environment also. The duration of all reactions or experiments is not the same. The reaction rate of the different molecules is changed after adding a catalyst. Any kind of physical change is not usually seen in the reaction.
FAQs
Q1. What is called a double displacement reaction?
Ans. The chemical reaction of this kind has positive and negative ions that exchange the position for forming a new compound. It mainly takes place in the iconic compounds and this reaction is presented as
Q2. What is the difference between single and double displacement reactions?
Ans. Only one compound is replaced by the other two reactants that rely upon reactivity in the single displacement reactions. The double displacement reactions guide to a reaction where reactant cations are exchanged as well as the anion too.
Q3. What is called the reactivity series of metals?
Ans. The reactivity series refers to a series where metals are organised for decreasing order of reactivity. Hydrogen is the only non-metallic element of this series for comparison purpose.
Types of Organic Reactions
Introduction
Various types of organic reactions are used in synthesising different organic compounds termed as organic reaction. These chemical reactions help to derive various important organic products which are further helpful in different pharmaceutical and industrial productions. These reactions are carried out by various processes like, addition, substitution, and some other reactions.
Chemical Reactions: Definition
Chemical reactions are simply associated with the mixing of two different chemical compounds that helps in deriving a final product. The substances involved in the reactions are called reactants and the resulting material is called the product. Chemical reactions are utilised in every course of human life.
In the course chemistry, a chemical reaction takes place when the bonds of the reactant molecules are disintegrated and new bonds are created formed for the molecule of the product.
Types of Chemical Reaction
The chemical reactions are subcategorised into two different sections based on the selection of reactant products; these reactions are termed organic reactions and inorganic reactions. Both of these types are mentioned briefly −
Organic Reactions
The reactions where products are organic in nature is known as organic reactions. In this course of reactions, one of the reactants gives away an electron pair to the other product where vacant valence shell is available. This creates a bond between the two organic compounds.
Inorganic Reactions
These kinds of reactions are widely observed in nature. These are mostly found in nature. These reactions are constricted mostly to the reservoirs because of chemical buffering between two inorganic samples. This kind of reaction is mostly observed in the case of carbonates and silicates.
Meaning of Organic Reactions
Figure 1: Five Types of Organic Reactions
Organic reactions are the chemical reactions involve the usage of organic substances. Organic reactions include different kinds of reactions based on the disintegration of the bonds found in the reactant molecules. Primarily there are four different kinds of organic reactions such as, substitution, addition, elimination, and rearrangement reactions. 80% of the organic reactions are carried out using these reactions path way. The substances involved in this reaction contain carbon atoms.
Types of Organic Reactions
There are various kinds of organic reactions to consider based on the treatment of the reactant substances and the breakage of bonds. It leads to the formation of different compounds are witnessed through various organic chemical processes. These chemical processes are mentioned below −

Figure 2: Addition reaction
No machine-readable author provided. Su-no-G assumed (based on copyright claims)., Electrophilic addition of Br2, marked as public domain, more details on Wikimedia Commons
Addition Reaction
Addition reactions take place when different substances take part in a chemical reaction to create an entirely new substance. In this course, the reactants found in the reaction do not lose any of the atoms. The addition reaction is quite a common procedure where the participant components have unsaturated C-C bonds found in double and triple bonds.
The presence of these double and triple bonds is found in alkene and alkyne. The weak π bond is transformed into two different strong σ bonds. This reaction is prominently observed when ethene and bromine are combined to produce 1,2- Dibromoethane. The resultant substance is colourless.
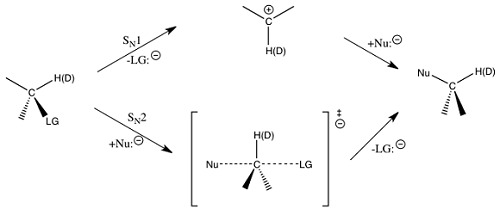
Figure 3: Substitution reaction
Aykutaydin, SN1 vs SN2, CC BY-SA 3.0
Substitution Reactions
In course of a substitution reaction, an atom or a conglomerate of atoms is substituted by another one or an entirely different cluster of atoms to create a fresh product. This reaction is also acknowledged as a displacement reaction.
Substitution reactions are widely used in different applications of organic chemistry. This kind of reaction is either categorised as nucleophilic or electrophilic in nature. It solely depends on the reagent that takes place in the course of a reaction.
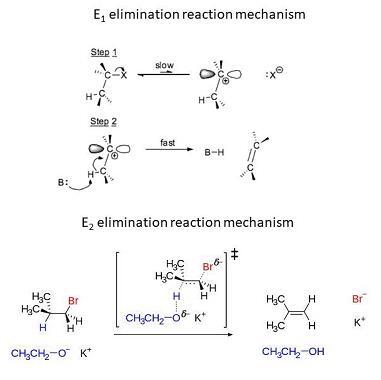
Figure 4: Elimination reaction
Kkukla, E1 Reaction, CC BY-SA 4.0) User:Innerstream, E2 elimination reaction, marked as public domain, more details on Wikimedia Commons
Elimination Reaction
Different reactions are involved in the case of elimination or removal of an atom or a cluster of it. As a result of elimination reaction various bonds are formed and it releases smaller units of molecules as a final product.
CH3CH2Cl→CH2=CH2+HCl
One of the prime examples of such a reaction is noticed in the conversion of ethyl chloride into ethene. In this course of a reaction, one unit of the molecule is liberated by HCl. It is formed by combining H+ which is derived from the carbon atom found on the left side of Cl- that is obtained from the carbon atom which is found on the right side of the reaction.
Conclusion
Organic reactions involve the combination of organic substances that are further helpful in different sectors. These reactions and its various subtypes are studied in the course of organic chemistry to assess the types and the associated pattern of the reactions.
Addition and substitution are two widely utilised organic reactions that help in deriving crucial end products that are helpful in industrial sectors. All of the components involved in this reaction require the presence of carbon atoms.
FAQs
Q1. What is Radical Reaction?
Ans. It is also called a radical substitution reaction which occurs by a free radical mechanism. This reaction results in the substitution of one or multiple atoms. It might even include the groups found in substrates liberated by different atoms of groups. This reaction includes three stages of the process and these are initiation, propagation, and termination.
Q2. What are nucleophilic addition reactions?
Ans. Nuclear addition reactions occur when nucleophiles that contain electrophonic double, triple or π bonds create an entirely new carbon centre combined with two units of single or σ bonds. The addition of a carbon hetero atom with nucleophile results in the variation of products.
Q3. What is an organometallic reaction?
Ans. This kind of reaction reflects nucleophilic characters of the carbon atom to bond with a metal. It helps in the development of the R-R compound that comes with a fresh carbon-carbon bond. This kind of reaction is widely noticed in the Ullmann reaction.
Types of Minerals
Introduction
Various types of minerals are defined as naturally occurring inorganic substances found in the solid form. The earth crust consists of different kinds of minerals such as; sand, coal, ore, or metal, as well as oil. Those consist of a well-defined chemical structure, crystalline structure, as well as formula.
Overview of Minerals
Minerals are substances that occur naturally on the surface of the earth. Those are typically solid in nature as well as inorganic substances. The minerals are formed through the geographical process and referred to as elements or chemical compounds. The nature of the minerals is normally crystalline and the process of geography helps in forming this crystalline form.

Figure 1: Minerals on the Earth
Minerals have a definite chemical composition as well as an ordered atomic arrangement. It looks like a bit of a mouthful but when it is broken down, it becomes simpler. These are called naturally occurring substances having an inorganic nature. It also has atomic structure, composition, and physical as well as chemical properties. These are extracted from the earth through the process of pumping, quarrying, or mining.
Minerals are seen as the most important part of everyday life and most of the earth is made through this substance. It can be presented as naturally occurring substances and have a crystalline structure. Some other organic materials are seen on the crust of the earth and those are diamond, gold, as well as silver.
Types of Minerals
The minerals are mainly divided based on their chemistry as well as the form of crystal. Those are classified into two main parts such as; metallic and non-metallic. The classified minerals are described below −
Figure 2: Types of minerals
Metallic Minerals
The minerals which metallic in nature have lustre appearance and these metals are seen in their chemical composition. These are called the main source of metals and extracted from mining. Some examples of these factors are manganese, iron ore, and bauxite. These are further divided into two categories such as; ferrous and non-ferrous metallic minerals. The presence of iron within the minerals is known as ferrous minerals.
Non-metallic Minerals
These kinds of minerals either present a non-metallic, lustre or shine in appearance. Those are called extractable metals and absent with the chemical composition. Some examples of those kinds of minerals include limestone, gypsum, and mica.
These non-metallic minerals consist of some features are as follows −
The metal of iron is produced from the iron ore and it does not exist in its pure form. It is extracted from the ore of iron by removing impurities.
The ore of bauxite is seen in the rocks that are deeply weathered. The volcanic rocks sometimes contain deposits of bauxite in some places.
Gold is considered as the most precious as well as the oldest element.
The ore of manganese is referred to as a silvery brittle or grey-white metallic ore found in many places.
Examples and Uses of Minerals
Some minerals are used in daily life and it improves the quality of everyday life. The minerals are gold, hematite, coal, as well as diamond. Coal is considered as a black substance which is hard. This substance mostly contains carbon and used as fossils fuel. In former times, it was used for running steam engines but, in recent times, it is used for producing electricity.
Gold is called a metallic mineral and found in the form of an element or an alloy with mercury or silver. It is mostly used as jewellery and stored as a value of currency. Hematite is referred to as an iron oxide bears the formula . Diamond is considered tome one of the forms of carbon and called one of the hardest substances found in nature. These are used as ornaments and some of them are used as cutters in the field of industries.
Difference between Metallic and Non-metallic Minerals
| Metallic Minerals | Non-metallic Minerals |
|---|---|
| These types of minerals have metals in their chemical composition. | The minerals do not have metals in their chemical composition. |
| These contain a shiny appearance. | These do not look shiny. |
| These are extracted from the igneous rocks. | The minerals are derived from the sedimentary rocks. |
| These have a ductile nature. | These are not ductile in nature. |
| These are malleable in nature. | These are non-malleable. |
Table 1: Difference between metallic and non-metallic minerals
Conclusion
Minerals are called the most usable substances in everyday life and formed by the geological processes. These are inorganic as well as solid in nature and called naturally occurring substances. The minerals are consisted of a crystalline structure and some examples are gold, diamond, coal, as well as rock salt. These are called the solid substances found in nature but these are not alive.
FAQs
Q1.What is the importance of minerals for humans?
Ans. The minerals, as well as vitamins, are important for the development of the body. Those help in keeping the human body fit as well as healthy and called the most useful source that helps in producing hormones.
Q2. What are the sources of minerals?
Ans. Some elements are responsible for making the minerals are iron, oxygen, calcium, copper, sodium, silicon, potassium, and many more. Some important minerals are quartz, feldspar, bauxite, cobalt, talc and pyrite.
Q3. What is the structure of minerals?
Ans. The minerals have seen an inorganic substance and found in the form of crystal as well as clear solid. It is produced through natural processes and also consists of a distinct chemical composition. These are identified through their crystalline structure, hardness, streaking and cleavage.
Types of Chemical Reactions
Introduction
Types of chemical reactions are classified on the basis of the reactivity of chemicals under different conditions of reactions. There are at least thousands of chemical reactions that occur everywhere. Only five or six of those are categorised, and most importantly one reaction can fall into many categories depending on different parameters. For getting more information about any kind of products in these reactions, it is important to understand those reactions in detail.
What is a Chemical Reaction?
In simple words, a chemical reaction is a chemical change that occurs to the starting materials and ends with a different kind of product. It involves the motion of electrons and leads to the breaking of chemical bonds and the formation of new products.

Figure 1: Representation of chemical reaction
The term chemical reaction is defined as the process, where the bonds of reactants break and a new product creates new chemical bonds with the help of product molecules. In a chemical reaction, the molecule or the particle reacts is called the reactant and the result of that reaction is known as the products.
Two types of changes occur in a chemical reaction, these are as follows −
Physical changes in a Chemical reaction
In a chemical reaction, the physical properties of the reactants can change such as the size or the shape of the reactants can change. For example, when a candle melts it changes its physical form.
Chemical changes in a Chemical Reaction-
In a chemical reaction, the chemical property or characteristics of the reactant can change too. For example, the density, temperature or energy of the substance can change. This type of chemical change is visible in combustion, rusting or fermentation.
Properties of Chemical Reaction
There are some unique properties of chemical reaction and these are as follows −
In a chemical reaction, two or more reactants react and as a result, it forms new more than one product.
The two main basic things of a chemical reaction are the product and the reactant.
In a chemical reaction, the chemical change brings physical changes with it, such as, the change of colour.
The reaction occurs in between the atoms, ions or molecules and results in breaking to forming the new bonds. It can happen without destroying any of the atoms.
Many factors decide the rate of the reaction, such as, temperature, pressure, or the concentration of the reactants.
Chemical Equations
Chemical equation is defined as the mathematical representation of a chemical reaction. It shows the product formation and states the conditions, on which the chemical reactions are dependent.
In a chemical equation, the reactants stay on the left side and the final products on the right side and these elements are connected with the help of one or two-headed arrows.
Different Types of Chemical Reaction
There are many types of chemical reactions, and the most important are as follows −
Combination Reaction
The simplest form of a chemical reaction is the combination. In this type of chemical reaction, two or more compounds react and combine to form a completely new product.
For Example,
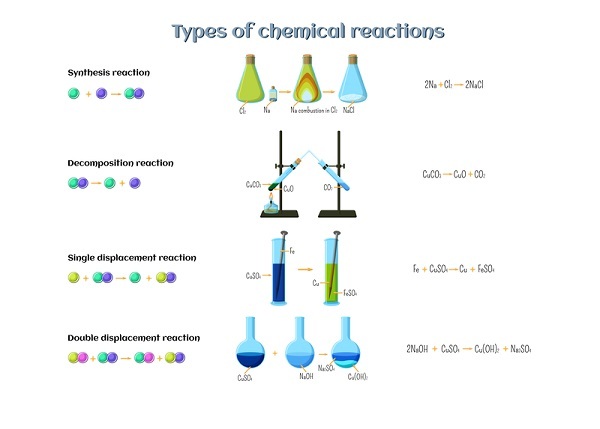
Figure 2: Types of chemical reaction
Combustion Reaction
This type of chemical reaction occurs between two reactants which are fuel and oxidant. The final product of this combustion reaction is oxidized.
For example, a chemical reaction between hydrocarbon (A) and oxygen
Neutralization Reaction
This type of chemical reaction occurs in between the base and acid where the final products are salt and water. The water forms with the help of OH- and H+ ions.
Decomposition Reaction
In this type of reaction, one reactant breaks into more products and to complete the process it requires a certain amount of energy. The energy can be heat, electricity, or light.
Redox Reaction
Redox reaction is also known as reduction and oxidation reaction. This process depends on the electron transfer, the reactant and the product either gains the electronic or losses. It has two types, oxidation and reduction. In oxidation, the reactant loose electron and in reduction it gains an electron.
Precipitation Reaction
In this type of chemical reaction, two solutions of salt are soluble and mixed which produces an insoluble solid.
Conclusion
A chemical reaction is a change in the nature of reactants to form a completely new product. The alteration can be in many terms, it can be a change in temperature, colour or energy or it can indicate the change of state. The reactants react chemically to form the new product known as the chemical reaction.
FAQs
Q1. What is the main cause behind chemical reactions?
Ans. The breaking of chemical bonds that occurs between reactant molecules or particles causes a chemical reaction. As a result of this chemical reaction, it forms a bond between the atom of the new product molecule or particle.
Q2. What is the importance of chemical reactions?
Ans. The chemical reaction can happen anywhere and to anything, even in living things, such as, in cells, animals, insects, and humans. It is important because it allows living things to evolve, grow, develop, adapt, and reproduce.
Q3. What is the main difference between the chemical reaction and chemical equation?
Ans. The major difference between the chemical reaction and the chemical equation is in their basic characteristics. The chemical reaction occurs between reactants to form a new product whereas the chemical equation helps to represent the chemical reaction with symbols.
Q4. What is a balanced chemical reaction?
Ans. Ans. The term balanced chemical reaction is used to represent those chemical reactions when both sides of the arrow in an equation, have an equal number of atoms.
Chemical Reactions for CBSE Class 12
Introduction
Chemical reactions for CBSE class 12 students are very interesting part of study. Itcan be seen everywhere in the daily human lives. It commonly happens when two or more compounds are changed into one or more substances due to the reaction between them.
When the chemical reactions produce the substances of chemical compounds or chemical components. In simple words, when the rearrangements are done within the constituent of atoms, the process is commonly called a chemical reaction.
Definition of Chemical Reaction
Chemical reactions are scientific phenomena happen everywhere all around the world. Starting from the metabolism of the human body to the way sunlight light impacts the environment; everything is influenced by the chemical reactions that are constantly taking place. It is defined as the phenomenon happens when the bonds between the molecules are broken and a new bond is formed for the formation of new products.
Physical and chemical changes in the product are result in by chemical reactions help to form a new product. In simpler words, the phenomenon of chemical reactions depends on the temperature, concentration and pressure of the reactants, especially while producing heat, changing colour, and precipitations during the reactions in real-time.
Types of Chemical Reaction
Chemical reactions are segmented into different types, based on the molecules, reactants and other factors like, temperature, heat production, and many more.
Combustion Reaction
This type of reaction happens between an oxidized product and an oxidizer in real-time. During this type of reaction, chemical needs an ample amount of fuel to burn. Combustion takes place in the presence of oxygen and produces carbon dioxide in return. For example, the combustion of magnesium metal is an example of a chemical reaction where two magnesium atoms react to the molecules of oxygen while releasing heat in the process.
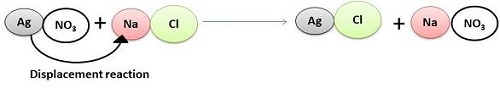
Figure 1: Displacement reaction
In the case of the displacement reactions a set of atoms is displaced by the other molecules. This type of reaction takes place when two compounds react with each other and as a result, the cations and anions switch their individual places for the formation of new products. For example, if the reactants are AB+CD in the first place, then the final result is CB+AD in real-time.
Neutralization Reaction

Figure 2: Neutralization reaction
The neutralization reaction is the reactions between acid and base while forming water and salt in a chemical process. Here, the molecules of water are formed due to the combination of OH- ions and the H+ ions during this reaction. For example, when sodium hydrochloride and hydrochloric acid react with each other, water and sodium chloride is formed due to a neutralization reaction.
Decomposition Reaction

Figure 3: Decomposition reaction
Decomposition reaction is a chemical reaction where a single component is broken down into multiple products at a time. The decomposition of calcium carbonate produces CaO, which can be a major example of the decomposition reaction in real-time.
Redox Reaction
The Redox reaction can better be defined as a reduction-oxidation process where electrons are transferred between the chemical species. For example, when zinc and hydrogen react with each other, an electrochemical cell like, redox reaction can be spotted.
Important chemical reactions for CBSE class 12
Sandmeyer Reaction
Figure 4: Sandmeyer reaction
This reaction is commonly seen during the reactions between synthesize aryl or alkyl halides from aryl diazonium salts. It can be used as a substitutionary method during the creation of diazonium salts in real-time instead of the aromatic amino groups.
Gattermann Reaction
In this reaction, chlorine and bromine are used as substituents of the benzene ring while preparing the benzene diazonium salt solution. In this reaction, a similar type of halogen acid can be seen present along with the copper powder in real-time.
Finkelstein Reaction
In the Finkelstein reaction, the alkyl iodides are prepared by a chemical reaction between Nal and alkyl chlorides with the help of a dry acetone.
Wurtz Reaction
This type of reaction is seen when the alkyl halides reacts with sodium using dry ether as solvent as solvent. During this reaction, hydrocarbons are extracted where the double number of carbon atoms can be seen within the alkyl halides. In this reaction, dry ether appears as a principal component.
Kolbe’s Reaction
According to Kolbe’s reaction, phenols generally react with sodium hydroxide in order to produce sodium phenoxide during the reaction. It further reacts with carbon dioxide under an acidic medium and furnishes salicylic acids as a final result.
Conclusion
The phenomenon of chemical reactions takes place when multiple molecules interact with each other for the formation of new products in real-time. Reactions like, Sandmeyer reaction, Gattermann reaction, and Finkelstein reaction are different types of the chemical reactions that help in the formation of new products. These reactions are common in everyday human lives; especially while manufacturing new products for daily life usage.
FAQs
Q1. Which factors impact on the chemical reactions most during the formation of new products?
Ans. Chemical reactions are deeply impacted by factors like, temperature, concentration, heat, and pressure of the reactants. During the reactions, the reactants face physical changes like heat production, colour change, and precipitation.
Q2. How many molecules are involved when chemical reactions take place in real-time?
Ans. The chemical reactions mostly take place between two or more molecules in real-time. The molecules need to have interactions between them during the formation of new products according to the laws of chemical reactions.
Q3. Which reaction is used in real-time for giving analogous compounds in the conjunction of two aryl groups?
Ans. The Fittig reaction is used during the preparation of aryl halides in dry ether medium along with sodium metal, especially while giving analogous compounds. This mostly helps in making bonds between two aryl groups via conjunction chemical reaction.
Types of Plastic
Introduction
Various types of Plastic are widely used compounds used in everyday life. These polymer units of long carbon chains societal impacts that not only is harmful to the environment but also the organisms associated with it. There are various kinds of plastics categorized based on their structure.
Definition of plastic
Plastic is a compound that was introduced to the world in 1846 by a German chemist Christian Schonbein. The term plastic is derived from the Greek word plastikos which implies to mould. This group of materials can either be synthetic or obtained from nature. It is generally soft, then hardened, and moulded to derive an entirely new shape of a compound.

Figure 1: Polyethylene structure
This substance like any other substance is made of molecules. Plastics however are prepared from larger blocks of molecules called polymer made up by long carbon chains. Polymers are always combined with different additives, like; plasticizers and colorants. These additives in turn, affect the chemical, mechanical structure, and properties of plastic. Polyethylene is the simplest form of polymer and most popularly used plastic.
Types of Plastic
All the plastics are made of polymer units of significant molecular weight. These substances are widely categorised into two subunits that are thermoplastic and thermosetting plastics.
Thermoplastic
These plastics can be easily deconstructed by the application of heat and the solid plastics of this form can be bent easily by the aforementioned process. These are made by linear polymers and further bonded with cross-linked polymers.
The most common examples of these plastics are PVC, nylon, polythene etc.
Thermosetting
These are the kind of Plastics that once moulded into a shape, cannot be softened any further shape by the application of heat. The polymers are heavily cross-linked fall under this category of plastics.
The most common example of these plastics includes Bakelite and melamine. Bakelite is utilised to make electrical switches and switchboards. Melamine is used to make harder substances like floor tiles.
There are different kinds of plastics based on with their materialistic constituents. These plastics are mentioned below −
Figure 2: Types of plastic
Polyethylene Terephthalate
Commonly called PET, this substance is used to make wrinkle-free fabrics. This is quite different from plastic bags that are obtained from various stores. These plastics can readily inhibit the settlement of oxygen molecules on the substance; also used in packing food and beverages.
Polyvinyl Chloride
It is a common example of synthetic polymer. Around 40 million tons of these substances are produced all across the world. PVCs are widely classified into two different types are rigid PVC and flexible PVC.
The rigid PVC is mostly used for various plumbing purposes and is utilised to make windows and doors. This material is also used to make cards for banks and manufacturing bottles.
Polyethylene
It is the most common form of plastic and also called polythene. The monomer of this component is called ethene. The density of this substance varies from 0.88g/cm3 to 0.96g/cm3. Both the high density and low-density polyethene are used for various packaging purposes. This substance has a low melting point.
Polypropylene
These are non-polar and partly crystalline polymers. The influence of heat can make this substance mouldable and transform it into a thermoplastic polymer.
Polystyrene
These substances are used in drain waste vent pipe systems and are also useful in making certain musical instruments. The density of this substance ranges from 1.06 to 1.08 grams. This plastic is often used.
Nylon
This product has high tensile strength. This product is quite lustrous and elastic. This substance is quite resistant to chemical substances. Nylon-6 is widely utilised in different sectors like aircraft and automobile industry".
Uses of Plastic
There are various uses of plastic. Few of them are mentioned as follows −
These have a sustainable and durable construction process.
It is used in automotive design and contributed to a multitude of innovations.
Plastic packaging is useful in the protection and preservation of various substances.
This help in making different components.
Harmful Effects of Plastic
Harmful effects of plastics are mentioned below −
Plastics are a major contributor to environmental pollution.
This substance also contributes to various harmful effects.
This product creates various harmful effects on health.
Plastic products harm the marine environment extensively.
Plastics are one of the major contributors to global warming.
Conclusion
Plastics are polymer compounds widely used in various processes. The longevity of the product marks it as a popular prodigy for various packaging processes and the production of final substances. Plastics are classified into various forms based on the constituent material and their respective traits. These are mostly classified into thermosetting and thermoplastic categories based on their ability of moulding.
FAQs
Q1. What is the difference between thermosetting and thermoplastics?
Ans. Thermoplastics can be moulded over the time by the application of heat. These are made of linear polymers. Thermosetting are the plastics are moulded once and cannot be back to its original shape.
Q2. What is the importance of plastic recycling?
Ans. Plastics should be recycled within the proper frame of time to prevent any case of plastic pollution that imparts adverse effects on the environment. These are non-biodegradable in nature and cannot be decomposed by microbes. Hence, biopolymers and biodegradable polymers are used.
Q3. What is the properties of plastics?
Ans. Plastics are generally ductile; these substances cannot conduct either heat or electricity. Plastics can be readily transformed into various shapes when heat is applied to them. Plastics are resistant to the effects of corrosion and are also resistant to different chemicals.
Types of Electrophoresis
Introduction
Types of electrophoresis are classification of the processes involves during application of electrical energy to any substance. Electrophoresis is derived from two Greek words, elektron means amber and phoresis means the act of bearing. In 1931, the first work of electrophoresis begins by Arne Tiselius. It was a primitively easy of separating the chemical and molecules analysis. The modern version of this process starts in the 21st century.
What is Electrophoresis?
Electrophoresis is a method and technique used for separating the RNA, DNA, and protein molecules. The whole process is done on the basis of size of the molecules and the electrical charge. For separating the molecules, one kind of electrical current is used and a gel medium is needed.
Figure 1: Application of Electrophoresis
Bensaccount at English Wikipedia, SDS-PAGE Electrophoresis, CC BY 3.0
The electrophoresis process is used in many sectors, such as −
Antibiotics are now one of the most used medical assistance to help patients in fighting the illness. Electrophoresis is used for antibiotic testing.
The most used sector of Electrophoresis is DNA analysis.
It is helpful in the vaccine testing.
Electrophoresis is used to analyse the proteins and the antibodies.
Principle of Electrophoresis
There is a separate principle of electrophoresis works on. The charged macromolecules are placed in the electrical field that helps to move the direction of the positive and negative poles. The movement is dependent on the macromolecules are already charged. For example, a nucleic acid is one kind of particle of negatively charged, it tends to move the direction toward the anode.
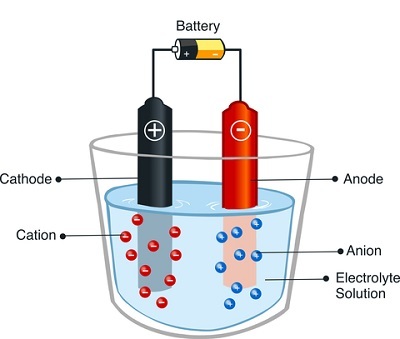
Figure 2: How Electrophoresis works
The two types of electrophoresis process are available, which are: capillary electrophoresis and slab electrophoresis. In the process of separation the protein, if it is already negative, then it moves to the anode and if it is positive then moves to the cathode. Scientists can measure the distance travelled by the molecules because smaller molecules can move faster than larger molecules and the size of the molecules is determined with help of logarithms.
The electrophoresis works on the basis of fundamental equation of electromagnetism, which provides it with a form, and it can be represented as,
Where F is the force, q is the electrical charge and E is the strength of electricity.
The equation simply means the higher charged particles have a stronger force when the electric field is applied. It means two molecules that have equal mass will move at the same time with different charges.
Types of Electrophoresis
The modern process of Electrophoresis has a supporting media, which helps to separate the charged molecules. It has two major functions, one is to sieve the molecule and the other is the absorption for separation. Two types of Electrophoresis method arementioned below as follows −
Capillary Electrophoresis or CE
This method required sub-millimetre diameter capillaries and micro and nano-fluidic channels. These analytes migrate through the electrolytic solution which influence and help to separate them on the basis of the movement of ions and partitioning into another phase.
Slab Electrophoresis or SE
This type of method is used to separate the protein. At a time, many samples are analysed with the help of 1D format. SE is much simpler method than the CE because, the CE needs proper instruments and set up whereas the SE needs nothing as such.
There are some other types of electrophoresis mentioned below −
Gel Electrophoresis- It is considered the most used process of electrophoresis. In this process, three kinds of gel are used such as: starch, polyacrylamide, and agarose.
Paper Electrophoresis- It is one of the simplest forms of electrophoresis. One kind of strip of paper is used in this method.
Zone Electrophoresis- It is used for the analysis of nucleic acids, biopolymers, and proteins.
Immunoelectrophoresis- This process is a combination method of electrophoresis and immune-diffusion.
Procedure of Electrophoresis
Electrophoresis of a DNA requires a few steps such as −
Preparation the sample.
In the next step the agarose TAE gel solution is prepared.
Gel casting is one of the most important steps.
Setting up the electrophoresis chamber.
The next step is Gel loading.
The process of Electrophoresis starts.
Observe the DNA and make notes.
The last step is to expose the ethidium bromide stained gel under UV light for taking a picture.
Conclusion
The electrophoresis method is completely laboratory-based, and referred to as the movement of colloidal particles. The gel is used in the process has pores which allow the smaller molecule of the mixture to move faster than the larger-sized molecule. The condition that is used in the process can be adjusted according to the requirement of separating the molecules.
FAQs
Q1. What is the basic principle of Electrophoresis?
Ans. The principle of electrophoresis is the migration of a changed species in a supporting medium, which can be fluid or gel. This occurs under the influence of an electric field”.
Q2. What method of Electrophoresis is used for DNA?
Ans. In the laboratory, gel electrophoresis is used for the DNA. It helps to separate them according to the molecular size from a mixture of DNA, proteins or RNA. These methods need an electrical field to get into the small pores.
Q3. What is the importance of pH in Electrophoresis?
Ans. The pH is important in electrophoresis because if pH level changes in the subject, then the structure and charge of the protein are also changed. It affects the whole process and the proper separation does not take place.
Q4. What is the difference between electrophoresis and immuno-electrophoresis?
Ans. Electrophoresis is a process of migration of electrically charged molecules through a gel base with the influence of electric field. On the other hand, immuno-electrophoresis is a method where the combination of protein electrophoresis and an antigen-antibody is required for separating the mixture of protein.
Types of Fabric
Introduction
Types of Fabric play a crucial role in society, as it provides numerous numbers of benefits to society. This acts as a vital component as the raw material used in the production or manufacturing of garments for all the civilians present within the society. The quality of fabrics tends to influence the quality of the materials used in making the garments. The quality of the garments also changes in the aspects of texture, and smoothness. Fabrics are widely used for a massive range of production purposes.
Defining Fabrics
Humans come across various kinds of fabrics on a daily basis. Fabrics are composed of quite long as well as larger wide sections of fibres with long strands of threads. However, fabrics are segregated into two different types involve synthetic and natural fabrics. Those have been derived from both natural as well as synthetic resources. Fabrics provide massive benefits to civilians as it proves to tear are quite flexible in nature and are easy in recycling and can be as well reused.
Types of Fabrics
Two major types of fabrics are found mostly used by humans; however, it is completely based on the sources of fibres they are produced from.
Figure 1: Types of fibre leading to the formation of Fabrics
Jan Široky, Barbora Široka and Thomas Bechtold, Classification of textile fibers, CC BY 3.0
Natural fabric is manufactured with the help of natural fibres. On the other hand, the synthetic fabrics are made out of man-made sources of fabrics. A categorisation has been provided in the above image that states the natural fibres are predominantly sourced from plants, animals as well as minerals. On contrary, man-made fibres are sourced from natural polymers such as milk fibres, as well as from synthetic source namely, acrylic, nylon and many more.
Natural Fabrics and its Types with Examples
Natural fabrics are derived from natural sources. For example, natural fabrics are collected from fibres, such as; cotton is collected from the cotton plant. More to this, silk is sourced from silkworm cocoons.
Figure 2: Various sources of natural fabrics
Natural fabrics, such as; cotton are made from plucking of seeds and thereafter spun. The image of the cotton plant is shown in the image above. Wool is quite a common natural fabric used from fibres are collected from sheep.
Synthetic Fabrics and with Examples
Synthetic fabrics are majorly manufactured from various chemicals are man-made or artificial in nature. Some of its types are rayon and nylon.
These are predominantly made from synthetic fibres where cellulose is synthesized. These fabrics are quite durable in nature and highly versatile in their usages.
Difference between Natural and Synthetic Fabrics
The natural fabrics are different from synthetic fabrics based on certain crucial factors. However, these include the factors such as; natural fibres differ in the aspect of warmth due to the little crips present within this fabric, whereas, synthetic or man-made fabrics do not cater to such qualities. Breathability is another vital notion quite noticeable in cotton as it supports the circulation of air. Moreover, natural fibres show strength with its strong layer of binding of threads. The man-made fabrics are durable in nature such as; polyester and spandex.
The other qualities that create a difference between natural and synthetic fabrics are, dying aspects, where linen seems to be the best as it does not fade away as easily as other fabrics does. The qualities such as; softness or scratchiness are also shown by wool and by the fabrics such as; silks. Lustre and elasticity are the two significant properties are responsible for creating a difference among natural and synthetic fabrics. Other property, such as absorption helps in segregating of both fabrics.
Importance and Application of Fabrics
Valid importance is noticed for the uses of fabrics as it is quite beneficial in various usages. The fibres are quite luxurious and provide a feeling of upscale. The fabrics are rich as well as intense in colour. Fabrics provide a wide variety of selection in their usages to humans depending on their purpose of usage. Fabrics are lightweight, reusable, and flexible in nature. Moreover, fabrics are tearing proof as well as these materials are easy to transport and storage purposes.
Conclusion
In this tutorial, the focus has been given on exploring what fabrics are and discussion has been conducted on their sources. It is noticed that natural fabrics are derived from natural sources whereas; synthetic fabrics are derived from chemical sources. More to this it is noticed that fabrics are quite essential as well as beneficial to mankind.
FAQs
Q1. What are the main types of fabric found mostly used by humans?
Ans. The main types of fabric that are found to be mostly used by humans include both natural as well as synthetic fabrics. The natural fabrics used involve, materials like wool, silk, cotton, linen which are derived from animals, silkworms, and plants like; flax and cotton. On the other hand, synthetic fabrics consist of nylon, rayon, acrylic, spandex, and polyesters are man-made.
Q2. What can be defined as fabrics?
Ans. Fabrics are defined as long and quite wider strands in the area of cross-section and are made up of fibres. These fibres are made up from sources of both natural as well as synthetic. Humans originally use natural sources for their wearable clothes other synthetic fibres are made out of chemicals.
Q3. What are the two ways used by humans to make fibres?
Ans. Two ways are used by humans to produce fibre; the first one is the process of dissolving the element of cellulose found in plants cell walls and reformed into threads. Secondly, it is made up from oil through the synthesis of cellulose.
Urea
Introduction
Urea is formed at the time of producing amino acid from dietary proteins. The chemical structure of the element is and another name of this element is Carbamide. The component is one of waste elements of living organism having no physical impact. The procedure of detecting the urea level helps to find the problems of kidneys in the body. The compound also has some commercial and agricultural values. The compound is used as fertilizer in the agricultural sector besides ammonia.
What is Urea?
Urea is a diamide state of carbonic acid and the chemical formula is . The component is mostly used in producing plastics, drugs, fertilizer, and feeds of supplementary products. This compound is known to exist in a crystalline structure and the compound has no particular colour. The boiling point of the component is 271F or 132.7°C. The process of breaking metabolic proteins in the bodies of all mammals helps to form urea as an end product. The compound presents not only in urine but also in blood, milk, bile, perspiration, and many more.
Urea: Structure
The compound is waste material of the body excretes from the body through along with urine. The compound has two groups attached with the functional group of carbonyl. The compound is not toxic and can react with water easily. The compound has no smell and no colour in normal form.

Figure 1: Structure of Urea
Jü, Public domain, via Wikimedia Commons
The two amide groups are connected with carbonyl group that connection helps to the formation of urea. The oxygen atom makes a bond with the carbon atom and makes a group of carbonyl. The Hydrogen atoms are connected with the nitrogen atoms and make the amide group.
Urea: Synthesis
Figure 2: Production of urea
The popular chemist in France named Hilaire Marin Rouelle had separated the urine and urea. Chemists are able to create urea by heating ammonium cyanate. The inorganic elements make a mixture and get fresh organic elements using laboratory synthesis process. The compound is produced in large number and produce liquid ammonia and carbon dioxide. Under high pressure and temperature liquid ammonia reacts with carbon dioxide (dry ice) to produce ammonium carbamate. Furthermore, ammonium carbamate reacts at high temperature and results in formation of Urea and water. The compound is made up of the combination of four hydrogen, one oxygen, two nitrogen, and one carbon atoms.
Urea: Formula
The chemical formula for urea is and the molecular weight is 60.06g/mol. primarily, this component is a colourless and appears in solid white colour. The density of the component is 1.32g/cm3, and the melting point is 133°C. Carbon dioxide and ammonia reacts at high temperature and pressure and results in formation of urea compound. The formation process of the compound is given below −
The compound has a high potential of consumption of nitrogen than ammonia and for that reason, it is used as a fertilizer in agricultural industries.
Urea: Properties
The compound is easy to find in different substances related to living body like, blood, milk, and sweat of mammals. Urea is made up with a mixture of oxygen, nitrogen and carbon. Urine contains the concentrated form of urea and with the other chemical elements. The compound can consume dry nitrogen with a quantity of 46%. The United State is one of the major producers of urea.
The main content for making fertilizer is hydrophilic in nature and consumes nitrogen more than other elements. The components are mostly used in making explosive elements, feedstock for animals, glues, highly expensive products, and plastics. The component is used for making the process of nitrocellulose explosive elements and used as a form of stabilizer.
Quantity of Urea production
The global production rate is 220 million tons on the base of every year. The demand for the compound is very high in the sector of agriculture. The other remaining part of the compound is used to resist urea-formaldehyde, production of barbituric acid, and to manufacture skin care products. Other chemical components like, carbon dioxide and ammonia are also actively involved in the production process of urea.
Urea: Usage
The compound is used in various ways like −
Pretzels are formed with the help of the browning agent of the component.
Melamine is produced with the help of urea.
The compound is able to find in stomach bacteria through tests.
The compound is used for making dish soaps, creams, and ointments.
Conclusion
Urea is the main discussable chemical in the above article and the compound is produces in the liver of the human body. The compound can be prepared artificially by using other chemicals. The synthesis process is discussed in the above article. The structure of the compound and demand for the compound in the industrial sector is discussed in the above article.
FAQs
Q1. On which places urea can commonly be found?
Ans. The compound is produced in the liver of the human body as a form of urine. Artificially it can be made in a lab with the help of ammonia.
Q2. Is Urea the pernicious element for humans?
Ans. The component has a very low toxic rate for all living organisms including animals and humans. The compound is pernicious in a form of ingestion for the birds and wildlife in the environment.
Q3. What is the difference between urea and Urine?
Ans. The urea exits from the body through urine and the compound is the main element between the waste elements of nitrogen. The compound is made from the liver with the help of ammonia and urine is made in the nephrons of the kidney.
Uses and Applications of The Noble Gases
Introduction
Uses and applications of the noble gases are very important aspect of chemistry to understand the contribution of these elements in our daily life. Noble gases behave like pure non-metallic compounds, as these are colourless and odourless gases with low melting and boiling points. In the liquid form, these elements are not good conductors of electricity.
The condition for noble gases to exist in their gaseous form is at standard temperature and pressure. The general configuration of the noble gases is considered as . The noble gases generally have a very stable electronic configuration that helps them to react at a very low rate.
What are Noble Gases?
Noble gases are usually the term given to the elements that exist in the 18th group of the periodic table. The elements are as follows: Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), and Radon (Rn).
It is necessary to keep temperature at standard state for noble gases to exist in gaseous phase. The noble or inert gases having a very stable electronic configuration and cannot form molecules readily, thus these elements are found in its mono-atomic form.
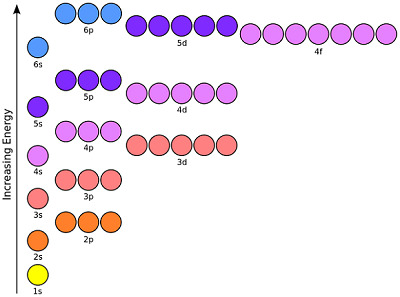
Figure 1: Electronic configurations
CK-12 Foundation, Orbital representation diagram, CC BY-SA 3.0
The group of noble gases are present at the rightmost side of the periodic table, approved by the International Union of Pure and Applied Chemistry (IUPAC). This group is populated with the non-metals. The elements are also called the neon group or the helium group after their residents. All the elements of the group possess very high ionization energies. The electrons of these elements are also equally distributed in their outermost shell. The density of the noble gases increases down the group, which results in bigger in size of the noble gas elements.
Noble gases: Properties
The properties of all the noble gases are given below −
Properties of Helium (He)
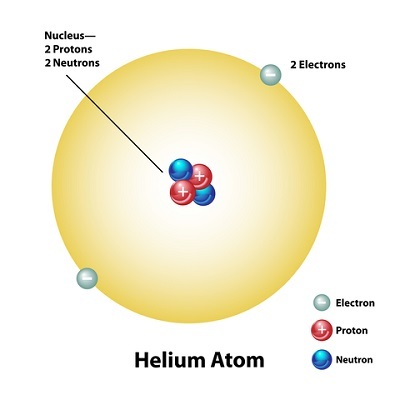
Figure 2: Atomic structure of Helium
Helium is also referred by its symbol He, and it possesses an atomic number of 2. At standard or normal Helium (He) exists in a colourless monoatomic gas with no distinct taste of itself. When used in concentrations of a small number Helium (He) acts in a non-toxic manner. It comes as the first gas in its distinct group and also the lightest of all of them. It is important to know that Helium (He) is an s-block element with an electronic configuration of 1s2.
Properties of Neon (Ne)
Neon is a chemical element which is also referred to by its symbol (Ne) and the atomic number of Neon (Ne) is 10. The standard conditions for temperature and pressure (STP) lets Neon (Ne) to stay in a colourless monoatomic state like the rest of the elements of its distinct noble or inert gas group. Neon (Ne) is the second-lightest noble gas just after Helium (He). It is placed to period 1 with also its inclusion to the group 18 of the modern periodic table.
Properties of Argon (Ar)
Argon (Ar) is considered to be the noble gas that comes at the third position with an atomic number of 18. Argon (Ar) is normally a colourless and odourless gas but when it comes in contact with an electric field it shows a violet or lilac-coloured glow. Argon (Ar) melts at approximately 83.81K.
Properties of Krypton (Kr)
Krypton (Kr) possesses an atomic number of 36. It also belongs to the group P like its predecessor Argon (Ar). Its melting point is around 115.78K and its electronic configuration is generally given as .
Properties of Xenon (Xe)
The atomic number of the 5th noble gas Xenon (Xe) is apparently 54. Its melting point has been approximately calculated as 161.4 K and its deductive electronic configuration is .
Properties of Radon (Rn)
The atomic number of the noble gas Radon (Rn) is 86. It is very important to point out that the element Radon (Rn) is radioactive in nature. Radon (Rn) shows melting and boiling points at 202K and 211.5K respectively and belongs to the 6th period of the periodic table.
Noble gases: Discovery
The noble gases or inert gases are not visible to naked human eyes and their nonreactive nature makes them very hard to spot and observe. In 1894, Sir William Ramsay did an experiment for discovery of noble gases. He tried to discard all gases from the air and thus he heated copper and magnesium and passed on air over it. It was observed that 1cm3 of air always stayed back when 100cm3 of air was used and that was how noble gases were discovered.
Noble Gases: Uses and Applications
The field of metallurgy has wide use for Argon, in providing the required inert atmosphere. The process of welding titanium, aluminium, stainless steel, and magnesium requires such an atmosphere. It is also regularly used when manufacturing titanium.
Germanium and silicon crystals require Argon in a little amount in them to produce electric bulbs and transistors.
Helium has the lowest boiling point among the noble gases, thus it is used in lasers to gain decreased temperatures. It has its applications in nuclear reactors as a cooling gas. The most common use of Helium is to fill up airships and hot air balloons.
Neon is applied in discharge tubes to produce a reddish-orange glow.
Conclusion
Noble gases or inert gases are commonly the name given to the elements present in the 18th group of the periodic table. Helium is the lightest among all of the gases and also has the lowest boiling point. The noble gases are highly non-metal in nature due to their low conductivity and electrical discharge, thus these gases are variedly used in the field of metallurgy.
FAQs
Q1. Can noble gases be found in solid structures?
Ans. The noble gases when subjected to the process of cooling or compression can be found in either a solid or liquid state. It can be referred to as solid-state when it’s in condensed form.
Q2. Is there any way helium can expire?
Ans. The noble gas helium doesn’t expire or degrade in its quality. It is required that it is stored in a cylinder with an airtight seal.
Q3. What leads to inhaling neon gas?
Ans. Neon gas is inert but it is termed as a simple asphyxiant. Inhaling it excessively can cause dizziness, nausea, vomiting, loss of consciousness, and even death.
Uranium
Introduction
Uranium was first isolated by the French chemist Eugène-Melchior Péligot, in the year 1841. This element was broadly used during the nineteenth century for various purposes like giving the glass a greenish-yellow hue which is also known as vaseline glass. In 1896, scientist Henri Becquerel first discovered its radioactive qualities. This discovery was further researched by Enrico Fermi in 1934. This research helped to discover the nuclear fission caused by uranium isotope U-235.
What is Uranium?
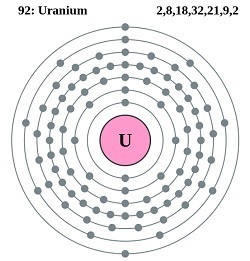
Figure 1: Atomic structure of Uranium
Pumbaa (original work by Greg Robson), Electron shell 092 Uranium, CC BY-SA 2.0 UK
Uranium is a radioactive element that is not very strong in nature. Uranium, is denoted by U and the atomic number of this component is 92. It is a heavy metal that has a high source of concentrated energy. It is a very common element like tin and tungsten and resides in various rocks in a 2-4 ppm concentration. It also has its presence in seawater.
Uranium: Discovery
German chemist Martin Klaproth first discovered uranium in the year 1789. He was scrutinizing pitchblende samples from the Joachimsthal silver mines when he discovered a uranium oxide. The mine was previously located in Bohemia and is presently located in Czechia. Uranium was first called uran by Klaproth. The name of this component was inspired by the planet Uranus.
Chemical Properties of Uranium
The chemical properties of uranium are the following −
It belongs to the Actinides group.
It is positioned in the 7th period and f-block in the periodic table.
The atomic number of this element is 92.
This molecule’s state is solid at 20°C.
The electronic configuration of uranium is [Rn] 5f36d17s2,
Its ChemSpider ID is 22425. It is a free database for chemical structures.
The melting point and boiling point of uranium are 1135°C, 2075°F, 1408 K and 4131°C, 7468°F, 4404 K respectively.
The density of this element is 19.1 g cm−3.
238.029 is its relative atomic mass.
234U, 235U, 238U are its key isotopes.
The CAS number of uranium is 7440-61-1.
Physical Properties of Uranium
The physical properties of uranium are the following −
The colour of the pure uranium is silver and it oxidises quickly in the air.
Naturally, the uranium ore is a mixture of its two isotopes. U-238 includes the 99.3% ratio and the rest in U-235.
Uranium is one of the heaviest naturally occurred elements on the earth depending upon the nuclei mass increase. The water is 18.7 times less dense than uranium.
Uranium has a range of isotopes and they exist in different forms.
Due to its high density, it can be used as an aircraft control surface counterweight and as a shield from radiation.
Almost 6.6 billion years ago uranium first evolved on this earth. It is uncommon in the solar system but it has slow radioactive decay that produces heat. This heat production often causes continental and convection drift.
Uranium: Applications
Figure 2: Applications of Uranium
Uranium is commonly used as a fuel for the generation of electricity in nuclear power reactors. It also produces almost 14% electricity for the world. The other main uses are described below −
Medicine: It is used as a radio element and gets injected into the human body to kill cancer cells in a specific organ.
Food Industry: It is used to sterilize the container and kill the pests, parasites and bacteria so that the food stays fresh.
Space Industry: Astronomers stay away from the sun in space and to produce heat uranium is a great alternative.
Industrial Sectors: Uranium gets used in x-ray, metallurgy, aeronautics and automobiles.
Culture and History: This component is used in archaeology to measure the age of a particular sample.
Effects of Uranium
The effects of uranium could be divided into three parts.
Effect on Miners
The people who are mining in the uranium mining sites come into direct contact with uranium. The safety measures that are taken for the miners are based on their financial suitability. It is seldom based on public health welfare. There the miners on the mine sites are directly exposed to the radio-isotopes that affect their health.
Effect on the Common People
The hazardous waste from uranium mining pollutes the environment. The consumption of hazardous wastewater or dust can cause common people various deadly diseases including cancer even though they are not directly exposed to it.
Effect on the Environment
The heat produced by uranium and its radioisotopes causes a continental shift. The plants that are grown over the tailing will produce vegetables filled with radioactive substances.
Conclusion
Uranium is rich in radioactive traits and hence is harmful to human health. Uranium was first used in war as a nuclear weapon. However, uranium has some useful applications as well. It can be used as medicine to cure cancer and as a sterilizer to kill bacteria and pests. Some automobile companies have discovered that for ammonia production, uranium worked as a better catalyst than iron. The nitrate of uranium gets used in a photographic toner. 6 or 4 is usually the valence of Uranium.
FAQs
Q1. How many years does it take for uranium to decay?
Ans. Uranium goes through a very slow radioactive decay. 4.5 billion years is the half-life for the uranium isotope U-238.
Q2. When uranium becomes safe?
Ans. Uranium and the decay products associated with it like thorium-230 and radium-226 will be dangerous and radioactive for more than a thousand years. However, after almost 500 years these products will become low-level radioactive.
Q3. What are the dietary sources of uranium?
Ans. Root vegetables like potatoes and beets have a lot of uranium present in them. Beef, eggs, milk, shellfish and fish also contain uranium.
Q4. Which place is at the top for uranium production?
Ans. Kazakhstan produces the highest amount of uranium in the whole world. In 2019, it produced 43% uranium. After Kazakhstan, Canada and Antarctica produce the highest amount of uranium. Uranium will last up to 80 years from now as per its current usage.
Uses: Iron, Copper, Aluminium, Zinc
Introduction
Uses of Iron, copper, aluminium, zinc, and many more are very important for our daily life usages as well as in industrial scale. Earth's crust is composed of several minerals and some of the metals and its ores are determined as the major resources for humanity.
Metals such as Iron, Copper, Aluminium, and Zinc are inorganic compounds that are found below the earth’s surface and are easily identified by evaluating their properties. These metals can be identified through their properties like hardness, toughness, ductility, malleability, elasticity, tensile strength, brittleness, and compressive strength.
What are p-block and d-block Elements?
Metals like copper, iron, and zinc are referred to as d-block elements and have different atomic numbers including Cu- 29, Fe- 26, and Zn- 30. The atomic number of Aluminium is 13 and this metal is generally placed in the p-block element in the periodic table. Metals can be divided into several types including ferrous and non-ferrous metals. Ferrous metals are cast iron and steel whereas non-ferrous metals are copper, zinc, and aluminium.

Figure 1: Non ferrous Metal Working Waste from Barton upon Humber
The Portable Antiquities Scheme/ The Trustees of the British Museum, Non ferrous Metal Working Waste from Barton upon Humber (FindID 434597), CC BY-SA 2.0
The metals that do not have any iron content are denoted as Non-ferrous metals. These metals do not get attracted to magnets and remain rust-free even if they get exposed to moisture. All these metals are good conductors of electric current, heat, and have wide applications in domestic as well as industrial fields based on their physical and chemical properties.
Properties of Iron, Copper, Aluminium, Zinc
Some common physical and chemical properties of Iron, Copper, Aluminium, and Zinc are stated below.
Metal like zinc and its alloys including brass and German silver has several commercial importances and is the major component of various electrochemical cells.
Aluminium, which is symbolized as Al, is the chemical element, a lightweight silvery white metal belonging to Group 13 of p-block in the periodic table. The abundant metallic element is generally identified as non-ferrous metals within earth's crust.

Figure 2: Common Metallic Materials
Zinc is referred to as the trace mineral that is usually present in red meat, poultry, and fish essential for the body to maintain metabolism, growth and development.
Copper is referred to as a chemical element that is symbolized by Cu with an atomic number, 29. It is identified as a soft, malleable, and ductile metal that has a higher rate of thermal and electrical conductivity.
Iron is identified as a strong, hard magnetic silvery-grey metal that is a chemical element with an atomic number of 26. It is generally utilised in manufacturing and construction fields mainly in form of steel.
Uses of Iron
Some common uses and benefits of iron (Fe) are stated below.
Iron helps in the preservation of several essential functions of the human body. Iron mainly provides gastrointestinal processes, general energy and focus, the immune system.
Iron also helps to maintain the body temperature and regulation of blood within the body. The major food sources of Iron (Fe) include fortified cereals, beans, lentils, tofu, spinach, dried fruits, prune juice, enriched bread, broccoli and nuts.
Iron (Fe) is utilized as a catalyst that is incorporated in the production of ammonia through the Haber process.
Cast iron can be determined as ferrous metals that are generally picked up by a magnet and are highly prone to rust when exposed to moisture.
Uses of Zinc
Some common uses and benefits of Zinc (Zn) are stated below.
Zinc is identified as the main component of electrochemical cells that is used in the manufacturing of electrical cells and batteries extensively.
The alloys of Zn including brass and German silver are used to make machines and industrial tools.
Zinc (Zn) can be used for the production of dyes, paints, anti-dandruff shampoos, and dietary supplements.
The zinc layer helps to protect other metals from corrosion. Zn is mainly used in hot-dip galvanization where galvanised steel is the best and cheap substitute in the case of stainless steel.
Uses of Copper
Some common uses and benefits of Copper (Cu) are stated below.
Copper has vast applications in plumbing and roofing materials.
Copper helps in the manufacturing of various musical instruments, circuits, PCBs, pipes, gutters, vaults, and doors.
Copper is the best conductor of electric current that is widely utilized in electric wires and cables.
Uses of Aluminium
Some common uses of Aluminium (Al) are stated below.
Malleable metals like aluminium are widely used in the production of utensils, as they can be transformed into flat sheets through rolling, moulding, and twisting processes.
Malleability is also determined as one of the properties of the metal that allows it to transform into flat metallic sheets when beaten. For example, aluminium sheets are utilized in the order to manufacture aircraft body, and doors, as it has high durability and strength.
Aluminium is used for producing utensils, as it is lightweight, malleable, and good conductor of heat and electric current.
Conclusion
All metals Iron, Copper, Aluminium, and Zinc look shiny and give the products a better appearance when polished with chemicals. As metals have a high durability rate, strength and deformability, they can be applied in various structural applications.
Metals can be found in both solid and liquid states. The metals that are present in a solid state are iron, copper, bronze, iron, and aluminium. In order to produce or manufacture several materials including satellites, cooking utensils, automobiles, satellites, and many more these metals are utilised widely.
FAQs
Q1. What is the major use of metals like aluminium, copper, iron and steel?
Ans. Metals are good conductors of electric current and heat energy. Metals like aluminium, copper, iron and steel are often used in order to make various materials like household appliances, electrical machines, aircraft bodies and doors, automobiles, and many more.
Q2. What is the necessity of Iron (Fe) in humans?
Ans. Iron (Fe) can be found in several food products including meat, fish and poultry. It helps to provide energy to the body, regulates blood pressure and body temperature, and helps to increase the level of haemoglobin.
Uses of Carboxylic Acid
Introduction
Carboxylic acids are prepared when the chloride of acid is hydrolyzed with a water base. This chloride is acid when reacted with the base they produce carboxylic acid, which can be acidified again through laboratory processes. On hydroxylation of acid anhydrides, R-COOH is formed artificially. Carboxylic acids are generally made up of 2 electronegative oxygen atoms that make the molecules of the acid polar.
What is a Carboxylic Acid?
The organic compound that has a carboxyl functional group is referred to as carboxylic acid. These compounds can be freely found in the environment and can be synthesised artificially by humans. Carboxylic acids get gradually deprotonate in order to form an anion of carboxylate. The anion of carboxylate has a general formula that can be written as R-COOH. Hexanedioic acid, one of the components of R-COOH can also be utilized in order to manufacture nylon-6,6. Carboxylic acids are often utilized widely in the production of several useful salts, which are included in soaps.
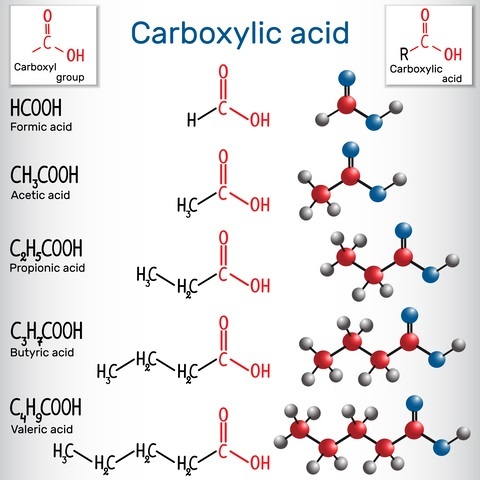
Figure 1: Structure of Carboxylic Acid
Carboxylic acids can be prepared through several artificial methods as mentioned below.
Primary Alcohols utilization for Preparing Carboxylic Acid
The aid of several oxidising agents including potassium permanganate ( for neutral, acidic, or alkaline media) and primary alcohols usually undergoes oxidation reaction in order to produce carboxylic acids. This acid can also be formed through the oxidation reaction of some other agents including chromium trioxide ( and Jones reagent), and potassium dichromate (– acidic media).

Figure 2: Oxidation of primary alcohol to carboxylic acid
Preparation from Nitriles
The formation of amides occurs when nitriles get hydrolyzed in laboratory methods. Amides then are incorporated into a reaction in the presence of the catalysts that helps in the preparation of carboxylic acids. In this reaction, this H+ or OH- generally acts as the catalyst. The amide stage reaction is terminated when mild reaction situations are used.
Prepared from Acid Chlorides
R-COOH is formed when the acid chlorides gets hydrolyzed with water. This hydrolyzation of acid chlorides with a water base compound generally creates ions of carboxylate that can be acidified in order to form the required R-COOH. On the either side, the hydrolyzation of Anhydrides with water base gives out Carboxylic acids.
Properties of Carboxylic Acid
Some physical and chemical properties of R-COOH are stated below.
The boiling point of R-COOH increases with the increased size of the molecules. R-COOH has a higher rate o boiling point if compared to alcohols and alkenes.
R-COOH are generally determined as a weak acid and can form hydrogen bonds, van der Waals dispersion forces, and dipole-dipole interactions with the molecules involved within it.
R-COOH have an average solubility rate in water as they are polar compounds and due to their hydroxyl in the carboxyl group, they form hydrogen bonds in H2O.
R-COOH is volatile in nature have a strong smell as found in vinegar. Vinegar generally contains ethanoic acid, butanoic acid, and rancid butter.
Apart from this R-COOH, esters have a pleasant and sweet smell that is used in the preparation of perfumes. The acids usually react with alcohol compounds to form esters.
Uses of Carboxylic Acid
Some major uses of Carboxylic acids (R-COOH) are mentioned below.
Carboxylic acids (R-COOH) contain several fatty acids that are highly beneficial for human health including Omega-6 and omega-3 fatty acids. The fatty acids help in cell membrane development and growth, control the use of other nutrients and maintain metabolism within the body.
R-COOH generally has fatty acids in them that are widely utilised in the production of soaps and detergents. Soaps are the potassium or sodium salts of Stearic acid and include a higher rate of fatty acids in them.
Figure 3: Carboxylic acid synthesis fom hydrazide catalyzed by copper (II) hydroxide
R-COOH is used in the food industries for the preparation of soft drinks, vinegar, and so on. These organic acids also have sodium salt that is used as food preservatives.
In pharmaceuticals industries carboxylic acids are often used in the production of medicines including aspirin and phenacetin.
The component of carboxylic acids like those that Acetic acids are widely utilized as coagulants in order t manufacture rubber, car tires, and many more.
The organic acids can also be applied in the preparation of dyestuff, perfumes and rayon.
Conclusion
Carboxylic acids contain a high amount of fatty acids including Omega-6 and omega-3 fatty acids that sometimes do not get well absorbed by the body. R-COOH helps the digestion process to slow down and absorption of nutrients and energy from food products. R-COOH can also be utilized as amino acids and acetic acids in food and pharmaceutical industries across the globe. The esters of R-COOH are generally referred to as carboxylates and when it is protonated its conjugated base produces carboxylate ion.
FAQs
Q1. What is the significance of carboxylic acids?
Ans. The component of Carboxylic acids like acetic acid is utilized as the coagulation that is incorporated in the production of rubber. This Carboxylic acid reacts with alcohol and forms ester that has a sweet smell used in the production of perfumes and scented cosmetic oils.
Q2. What are the chemical properties of carboxylic acids?
Ans. The carboxylic acid can be reduced to alcohol when it is treated with hydrogen and passes through a hydrogenation reaction. The α-carbon of R-COOH can be halogenated through the Hell-Volhard-Zelinsky reaction. The compounds of R-COOH get converted into amines through the Schmidt reaction.
Q3. What are the major applications of carboxylic acids?
Ans. Carboxylic acids are utilized in the preparation of car tires, rubber materials, cosmetic oils, perfumes, and textiles. Hexanedioic acid, one of the components of R-COOH can also be utilized in order to manufacture nylon-6,6. Ethylenediaminetetraacetic acid can be used as a chelating agent in several laboratories.
Uses of Acetone
Introduction
Acetone stands for a compound that is organic in form and the chemical identity of this compound is . This application of this element is mainly seen as an additive for removing nail polish. It is dissolved in paint and it is mostly used for starting up of diesel engines.
What is Acetone?
Acetone refers to the smallest as well as simplest members of the family of ketone and it is also called as propanone. No specific colour is seen in this liquid, the characteristics of this compound are a little volatile, and it is highly flammable. A wide application of this compound can be seen in the manufacturing process of the different products.
Figure 1: Structure of acetone
Acetone can be getting by heating the anhydrous calcium acetate in a laboratory. It is naturally seen in the environment but it can be produced in the laboratory. Pure acetone includes its molecule and it can break into superglue and leaves when it is denatured with alcohol. The molecule of this compound contains atoms of oxygen, hydrogen and oxygen.
Properties of Acetone
Acetone is a member of the ketone group and it is the smallest of them.
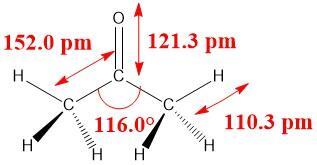
Figure 2: Structural formula of the acetone molecule,
The chemical identity of this element is the molar mass of this compound is 58.08 g/mol.
The density of this element is very low like 0.784 g/cm3 and it easily dissolves in different liquid substances such as water.
The point of freezing this compound is −94.7°C and it takes 56.05°C to boil.
The flashing temperature of this element is −20°C and the IUPAC identity of this element is Propan-2-one.
The dissolving power of this compound is very high and it is dissolved in every liquid such as different types of fats, resins as well as cellulose acetate. This element also dissolves in nitrocellulose along with cellulose ethers.
Daily Life uses of Acetone
Acetone is used widely for different purposes of the daily life schedule and some of them are mentioned below.
Acetone is applied in the production process of several compounds like chloroform, sulphonal, artificial scents as well as different cordites.
The compound is widely applied in the process of extraction of some important oils for daily use.
It is widely applied in the making process of different household products, especially in some cosmetics and beauty products.
It is sometimes used as a solvent for washing the glass apparatus.
The application of this compound is seen as a cleaning particle that is useful in removing some harsh grease that range from different fabrics.
Acetone is also used in polishing engines of the automobile sectors or other types of vehicles.
This element is very useful in polishing shoes and it makes the shoes clean and shiny.
It is helpful in deep cleaning the keyboard and other stubborn marks on the windows made of glass or metal.
Acetone is used in the making process of some beauty products like sanitiser.
Acetone is widely used in the medical field in recent times and it is used in the production of pills, different types of tablets along with liquid medicines for the maintenance of appropriate density.
It is one of the most important components in rubber cement as it builds a strong bond that guides to set the crack quickly.
It is widely applied in the making of glues, pastes as well as adhesives as it quickly dissolves the substance’s surfaces. It is sometimes used as a thinner for different products.
Acetone is also made from sodium bicarbonate along with red phosphorus. These both compounds are very volatile in nature so in the preparation of some explosives it is used.
Industrial Uses of Acetone
Acetone is one of the most essential ingredients in different industries and it has some factorial applications as well.
Acetone is applied like a solvent for various liquids like different types of celluloid, varnishes as well as lacquers.
This compound is added to the natural compressed gas fuel in different mineral oils like petroleum for providing them with a better thickness.
It is used as a remover of oil content from the surface of the water so that is the purpose of saving marine lives it is widely used.
Acetone is applied for the safe transportation process, as it is highly flammable to the chemical fuel such as acetylene.
It is used severally in the electronic industry for the clearance of some electronic gadgets.
Conclusion
The storage process of acetone is highly secured and it is mainly stored in an airtight jar because it has a higher flammable power. The storing places of these compounds must be far away from any burning agent like gas stoves or burning ovens. This element should be used by wearing chemical safety goggles. Gloves, boots as well as aprons should be used when acetone is used in the laboratory as it has a higher flammable power.
FAQs
Q1. What is the production process of acetone from acetyl chloride?
Ans. The production process of acetone from acetyl chloride with the application of dimethyl chloride gives acetones along with cadmium chloride. The reaction is presented as
Q2. What is the production process of acetone from isopropyl alcohol?
Ans. The production process of acetone from isopropyl alcohol starts by passing water vapours of isopropyl with the copper catalyst at the temperature of 573K. The chemical reaction is presented as
Q3. What are the safety tips for using acetone?
Ans. The using area need to be well ventilated and the atmosphere need to be dry as possible for using this compound. It is very important to keep a fire extinguisher during the application of acetone and windows must be opened as well. Smoking or burning any flammable substances is strictly prohibited during the application of this compound.
Uses of Citric Acid
Introduction
Citric acid is produced organically and is readily found in nature, present in many fruits and vegetables. Lemon is an example of a citrus fruit that contains the largest amount of citric acid. Citric acid is commonly used by food processing companies in various food and beverages as an acidulant. It is mainly used in so many varied ways due to the way it is prepared, that is by the process of Fermentation. Its usage is not just limited to food industries but other industries like cleaning and cosmetic product manufacturing as well.
What is Citric Acid?
Citric acid can be defined as a tribasic weak acid, which is available in two major forms - monohydrate and water-free (anhydrous). Its chemical formula is . The chemical formula according to its molecular structure is given as . It is considered an intermediate product in the citric acid cycle according to the subject of Biochemistry; the said process is exclusive to the metabolism of all aerobic organisms. Citric acid is considered really advantageous as it is readily found in many fruits and vegetables.
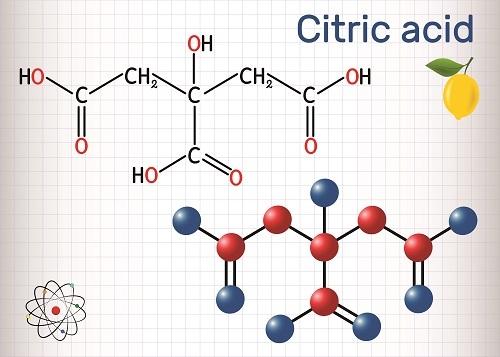
Figure 1: Structure of Citric Acid
Citric acid has a sour taste to it and is completely odourless. In its solid state, it is found as a white crystal, and is also monoclinic. Due to its existence as a tribasic acid, it is not an easy process to develop acidic salts of Citric acid, it needs to be done with very much caution by controlling the pH levels so that the compound doesn't transform into a crystal.
Citric Acid: Preparation
Figure 2: Preparation of Citric Acid
The extensive process of preparation of Citric acid is done through 5 steps. They are explained below −
Step 1
Firstly, a decent amount of citric acid in its crystal form is required, with an additional 450 ml of lemon juice. The lemon juice should show a pH level of around 2 or 3 on a scale of 1 to 10.
Step 2
Sodium hydroxide is required for the process so it is advised to add some eye drops which contain approximately 10% of it. The solution now needs to be retested. After pouring it into a second container it needs to be observed if there are any traces of solid particles in the solution.
Step 3
28 grams of calcium chloride with 70 mL of distilled water needs to be added to the solution in a glass and boiled.
Step 4
The solution now needs to be filtered again to remove any traces of calcium citrate. It needs to be combined with significantly diluted sulfuric acid and stirred properly The solution now needs to be filtered again and the citric acid needs to be separately kept in a beaker in presence of water.
Step 5
The whole thing now needs to be heated over medium heat. This needs to happen until the evaporation of the water that is present in the beaker. The citric acid now needs to be removed and cooled.
Citric Acid: Benefits
Citric acid leads to the formation of a varied range of metallic salt, which includes the combination with copper, iron, manganese, magnesium and calcium. Citric acid is used as a sequestering agent in various commercial processes owing to these salts and is also known as an anticoagulant blood preservative. These salts are also the reason for their antioxidant properties.
Citric Acid: Uses
Citric acid is used in many different fields owing to its varied properties, they are as follows −
Food Additive
Citric acid is heavily used by food processing companies as a flavouring agent due to its sour taste. It is also used as a food preservative. In various beverages like soft drinks and also in syrups citric acid is used owing to its properties as an acidulant. White-powdered form of Citric acid is applied in the production of sour candies. It is also used as an emulsifier to produce ice creams by various companies, to chase away fat globules.
Cleaning Agent
Citric acid has chelating properties which is why it is necessarily used in cleaning products. It is also productively used to clean away limescale from evaporators and boilers. Owing to its properties of softening the water it is commonly used in soaps and laundry detergents. Due to its overall cleaning properties, it is used majorly to produce household cleaners for kitchens and bathrooms. It also fulfils the purpose of a deodorizer.
Cosmetics
Citric acid can result in discarding dead skin from the body, thus it is heavily used in home masks. It leads to the improvement of the tone of the skin and also in its growth, which results in the removal of wrinkles. Citric acid is very useful in balancing pH levels thus it has its usage as a cosmetic ingredient, according to its requirement.
Industrial Uses
Citric acid is commonly used in commercial purposes for the manufacturing of detergents, electroplating and also leather tanning. Due to its curing properties citric acid is variedly used in pharmaceutical industries as well.
Conclusion
Citric acid is considered to be really advantageous for human benefits as it is readily present in many fruits and vegetables. It is extensively prepared through a 5 step process. Citric acid produced metallic salts that are used as a sequestering agent for various commercial purposes.
Citric acid is mainly used by food processing companies as a flavouring agent and is also used as a food preservative. In various beverages like soft drinks, it is used owing to its acidulant properties. It is also used in the cosmetic, pharmaceutical and cleaning products industries.
FAQs
Q1. What can drugs produced with citric acid cause?
Ans. Drugs produced with the help of citric acid are not recommended. It can lead to confusion, light-headedness, chest pain, fast heartbeat, pain, tingling or numbness in hands or feet, and fatigue.
Q2. How long can citric acid last?
Ans. Citric acid has a shell life of three years since it is being manufactured. It needs to be placed in its original container only, with cool and dry conditions.
Q3. Can citric acid be used as a disinfectant?
Ans. Citric acid possesses the properties of a disinfectant. It kills bacteria mould and mildew, that's why it is used as a proper disinfectant.
Uses of Coal
Introduction
Coal, is also known as Black diamond. Coal is inflammable black or brownish alluvial rock with a high amount of carbon obtained. Coal can take more than a thousand years to be formed, so coal is also considered a non-renewable energy source.
In the current world of digitalization and technology-based development, coal appears as an effective source of energy for not only industries but also households. Coal is of four types lignite, subbituminous, bituminous, and anthracite. The footfall of coal is in almost everywhere that depends on the generation and usage of energy.
What is Coal?
Figure 1: Structure of coal
real name: Karol Głąb pl.wiki: Karol007 commons: Karol007 e-mail: kamikaze007 (at) tlen.pl, Struktura chemiczna węgla kamiennego, CC BY-SA 3.0
Coal is a rigid (solid) carbon-rich inflammatory substance that is also known as black diamond. The main chemical element of coal is carbon and also has some other chemical substances like hydrogen, sulphur, oxygen, and nitrogen. Coal is expressed as a high carbonaceous substance which is made of more than 55% by weight, made of thickness and hardening of the innovative leftover of plants as a deposit.
Composition of Coal
Cellulose, hemicellulose, and lignin are present in the plants and from these material coals are made. The main part of coal is the lignin of the plants, and has cellulose and hemicelluloses condensation is also altering from more than 4% to 39%. Sometimes also gets some wax, nitrogen- and sulphur-obtaining chemicals, and other organic elements are also being seen.
Lignin contained approximately 54% carbon, 6% hydrogen, and 30% oxygen though cellulose is contained 44% carbon, 6% hydrogen, and 49% oxygen. Bituminous coal makes up nearly 84.4% carbon, 5.4% hydrogen, 6.7% oxygen, 1.7% nitrogen, and 1.8% sulphur. It means when the coal is processing must expel the majority of the oxygen and hydrogen and the only emit of carbon is known as carbonization.
Formation of Coal
Dehydration, decarboxylation, and demethanation are the primary stage of carbonization. Dehydration is the method of emitting water elements from ripe coal by reactions. The reactions are mentioned in below.
Decarboxylation is a chemical response that omits from ripe coal .The reaction is below.
Here is the demethanation process, where methane is eliminated.
Above-mentioned processes are usually considered extreme analysis processes. It expressed when the coal got heated then the volatile portion is redemption.
Coal: Properties
Coal is a non-classic alluvial fossil rock, which is black or brownish in colour. Moisture, moisture is a very important property of coal. Volatile is another vital property of coal because when coal got heated that time methane is released. After coal is burnt, it becomes ash which gradually becomes biodegradable .A permanent number of carbons is present in the coal but also has some other composites like, hydrogen, sulphur, and oxygen.
Coal: Uses
Human beings used Coal in various fields. There are mainly two types of fields are present, one is household and the other is commercial.
Household
Until now, so many villages are present in these worlds that do not have a gas connection. Therefore, people are dependent upon coal for cooking, and to make fires. Some winter seasonal countries are used coal to increase the room temperature.
Commercial
Coal is hugely used in the commercial field. Some are below mentioned.
Producing Electricity
Coal is usually utilised in thermal power production, which is help to electricity produces. The coal dust is burn at extreme temperatures, which further changes water into dampness. This dampness is utilised to move the turbine wheel at a high speed in the presence of high magnitude field to generate electricity.
Steel Industry
Coal is not utilising directly to make steel. First, here coal coke is creating which is made from boiler coal. Producers used coal coke and smelt iron ore into iron. After this, it is used to make steel. In producing of steel, ammonia gas is recuperated from coke burners and used to make nitric acid, ammonia salts, and fertilisers.
Figure 2: Uses of coal
Industrial Use
Cement industry, paper and aluminium industry are utilised the well-known industries which make some particular items. Also, include chemical and pharmacy industries.
Gasification and Liquefaction
Synthetic gases are mainly generates from coal because coal can be a proselyte into the composition of carbon monoxide and hydrogen. These gases are interim products that can be further changed into various types of products such as urea, methanol, authentic hydrogen and so on Coal is also revolves in fluid which is called synthetic fuels. Aspirins solvents, soap, dyes, and plastics and fibres along with nylon and rayon are some commercial products, which are making from coal.
Conclusion
Coal is a hugely utilised material, which can get from the environment. Too many uses of coal have also side effects. The main source of energy in Commercial fields is coal to manufacture any type of product. Coal is produce by the carbonization method. Mainly, three stages are present in the carbonization process. These are Dehydration, decarboxylation, and demethanation.
FAQs
Q1. What types of substances can be extract from coal when it is use in industries?
Ans. Coal gives various substances such as benozle, coal tar, sulphate of ammonia, creosote, and so on. Generally, in industries coal is use as the main origin of energy.
Q2. What are the common properties of carbon-rich coals?
Ans. Generally, carbon-rich coal is black or brown in colour and it is situates in an alluvial layer in the ground. In the present days, it acts as a vital fuel that is collect from the ground as a fossil fuel.
Q3. What is Decarbonisation?
Ans. The word Decarbonisation means the deduction of carbon from any type of elements or components. The exact meaning of the word is the transformation of an economic system that sustainably decreases and recoups the outflow of .
Uses of Boron and Aluminium
Introduction
Uses of Boron and Aluminium are mainly consists of applications in textile sector and in the preservation of wood. Boron is a very uncommon compound in this world because of the trace formation of stars and the big bang in the solar system. The Atomic number is five for boron in chemistry and the aluminium atomic number is thirteen.
Aluminium is available in igneous rocks as micas, feldspars and feldspathoids in form of aluminosilicate. The hydrate aluminium oxide is the primary element of bauxite from there aluminium is mainly formed. Boron is commonly found in nature as a form of boric acid and boric acid.
What is Boron?
Boron is a black colour element that is a lustrous metalloid, crystalline in nature. The compound’s chemical symbol is B and five is the atomic number of this element. The compound can alter its colour from black to brown at the time it was amorphous in the state.
The element has five protons and amount of neutrons is six and the number of electrons amount is five. Valence shells need some electrons and it also helps to create a covalent bond. The borax in mineral form, boron carbide and boric acid is formed from the reaction.
Boron: Structure
Boron consists of protons in the amount of five and all are charged positively. The neutrons quantity is six and they formed the nucleus of the compound. The elements of the electrons are surrounding around the cells and are available in the shells of electrons.
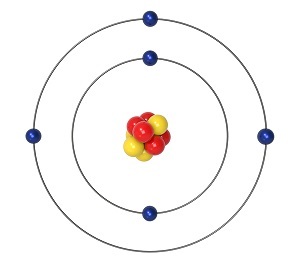
Figure 1: Atomic structure of Boron
The properties of the compound that are chemical and physical are stable for the electrons. The element can be found in the periodic table’s second number and the chemical group number is thirteen. The compound is available in block number P in the periodic table.
Boron: Properties
The chemical and physical properties are discussed below in detail.
The point of boiling of the element is 4275K and the element’s melting point is 2365K.
The potential of ionisation is -8.298eV and 10.811u is the atomic mass of the element.
The electronegativity of the element is 2.04.
The element can make two stable isotopes and they are 11B(19.9%) and 10B(80.1%).
The element's rate of oxidation depends on the crystallinity, purity, temperature and size of the particular element. The element reacts in the presence of air and temperature in a proper way for reaction. The element is burning itself in the presence of temperature and boron trioxide is the result of the reaction.
Hydrochloric acid and hydrofluoric acid are highly reactive with the elements with the crystalline form of the element. The elements slowly react with hydrogen peroxide and nitric acid in concentrated form.
Boron: Usage
The element is used in various forms like, borates, borazine, boric acid etc. The usage of the elements is described below −
The element is utilized to obstruct the reaction between nitrate as well as aluminium and also obstruct the formation process of amide.
The compound is used to make glasses and ceramics.
Perborate is made from the element that is used majorly as a detergent.
The elements are used for margarine and fish preservation.
The elements are used in the textile sector and it also used in the preservation of wood.
Definition of Aluminium

Figure 2: Atomic structure of Aluminium
SM358, Public domain, via Wikimedia Commons
The element is quite silvery in colour and the chemical number of the element is thirteen. The element can be found in nature in various forms but not in metal form. The element can be found in animal bodies, rocks and many more. The chemical name of this element is AI and the element's name comes from the Latin word. The word alumen represents another form and that is called aluminium potassium sulphate. The chemical formula of the element is and potash alum is another name for this element.
Aluminium: Properties
The element is made from a sixteen-kilometre crust of the earth with a concentrated state and keeps 8% weight at the time of its formation. The two compounds like, silicon and oxygen are the two elements that are close to aluminium. The properties of the elements are described below −
The power of the atom in the element is 13 and the weight of the atom is 26.9815384
The point of boiling is 2467 degrees and 2.70 is the gravity of the element.
The valence of the element is 3 and the chemical name is aluminium.
Aluminium: Usage
The usage of the element is described below −
The elements are used to produce foil, coil, cans and other packing materials.
The element is used for preparing bodies of cars, spacecraft, aircraft, cycles and many more.
The elements are also used for making home appliances.
The making of alloy that is used for coins is also made of aluminium.
The element is also used for preparing paints, wires and reflective surfaces.
Conclusion
Boron and aluminium are the main discussable things in the above article. Boron is a very uncommon element and five is the chemical number of the element. Aluminium is commonly used for producing home appliances and various purposes of industry. Both elements have very high commercial values and both use hugely for preparing daily usage and industry-related products. The properties of both elements are discussed in detail in the above article. The structure and uses are also discussed in the above article.
FAQs
Q1. What is the main usage of the Amorphous boron?
Ans. The Amorphous boron is commonly used in the production of rocket oil where it acts as an igniter. In addition, they can also be used in the generation of pyrotechnic flares as they can give flares of distinctive green colours.
Q2. How Trihalides is formed?
Ans. Trihalides is formed as the result of the halogenation procedure. The reaction involved in the formulation of Trihalides is
Q3. What are the elements needed for making aluminium?
Ans. The element is made with the help of bauxite and aluminium oxide. The other process is to apply aluminium oxide with 50% amount and bauxite in high quality.
Uses of Borax
Introduction
Borax is a type of natural mineral and one of the most signification compounds of boron. Its chemical formula is that is present in both anhydrous and decahydrate states. It is available in hand soaps, laundry boosters and sometimes in toothpaste. The products are powdered hand soap, 20 Mule Team Borax and tooth bleaching formulas.
What is Borax?
Figure 1: Structure of anhydrous Borax
Borax is a solid crystal type of boron compound that is also called sodium tetra borate or sodium borate. This compound easily gets dissolved in water and it is soft and colourless. Borax has one borate ion and 3 positive ions. There are various almost similar minerals like decahydrate, pentahydrate and octahydrate salts that are also known as borax. Below is the list of the formula for these borax salts −
| IUPAC Name | Formula |
|---|---|
| Sodium Tetraborate (Anhydrous) | |
| Sodium Tetraborate pentahydrate | |
| Sodium Tetraborate octahydrate | |
| Sodium Tetraborate decahydrate |
Table 1: Different Types of Borax Minerals
Borax: Discovery
Borax was first got discovered in the year 1856 in the Tehama County of Northern California in North America. The official discovery of Borax had first happened by Aaron and Rosie Winters. They found it as they dropped alcohol and sulphuric acid into ore in the Death Valley. Mining started happening after the year 1880.
Properties of Borax
Borox has a few distinctive chemical and physical properties. Both properties of Borox are discussed below.
Chemical Properties
Borax is highly flammable as well as the colour of its flame is yellow-green. It has a high solubility in ethylene glycol and a low level of solubility in acetone. Borax gets easily transformed into boric acid by reacting with acids. The reaction is −
It reacts with sodium hydroxide. The reaction is −
Physical Properties
Borax is a white colour or colourless solid compound that is easily soluble in Aquas. The molar mass of borax is 202.22 when in an anhydrous state and 381.38 when in a decahydrate state. It has a density of 2.4g/cm3 and 1.73 g/cm3 in the anhydrous and decahydrate state respectively. Borax has a melting point of 743°C in an anhydrous state and 75°C in a decahydrate state. The boiling point of its anhydrous state is 1575°C.
Borax and Boron
Figure 2: Borax and Boric acid
Borax and boric acid both are made from boron. Borax is a type of salt that helps to create boric acid. It is a type of natural mineral that is present in the mines. It can be collected from evaporating deposits. Boric acid (H3BO3) is the purified form of borax. Borax and boric acid both can be used for insect control and creating slime purposes.
Uses of Borax
Borax has various useful applications in the modern world and different industries. Some of the major uses are −
Borax can be used as an alkali by photographic developers.
It is a widely used compound for metallurgy.
It can be used for food decoration purposes by using it as a texturing agent.
It can be used to kill fungi for its antifungal properties. It helps to cure different types of foot infections due to this quality.
It helps to cure thrush which is a bacterial infection that happens to horses.
It helps to treat timber and other wood products that are infected by woodworms.
Some detergents and cosmetic products also contain borax.
It can be also used as a flame retardant.
Borax can be used both as an insecticide and as a fertilizer.
Borax is used for vitamin supplement production. It is an important chemical substance that is required in the body.
It is used in the forge welding process widely by blacksmiths.
It can be also used as a preservative in taxidermy.
It is used in swimming pools to maintain their pH level.
This compound can be to create teeth bleaching products and enamel glazes.
Borax gets used in glass, ceramics and pottery-making purposes.
Effects of Borax
The sodium tetraborate decahydrate form of borax is not acutely toxic and U.S EPA stated it is a moderately safe chemical compound in the year 2006. Following are some benefits caused by the use of borax.
Borax helps to prevent arthritis.
It can be helpful for the healing of sore tongues and swollen mouths & throats.
It helps to cure swollen red eye.
It cures urinary infections and menstrual problems. In addition, it helps to deal with any sort of womb inflammation.
It increases both male and female libido.
It helps in cancer treatment.
However, borax has some health risks as well. Long-time exposure to borax can cause diarrhoea, vomiting and nausea. Long-time exposure can also cause infertility and cancer, problems that can be treated only by the measured exposure to borax.
Conclusion
Borax is a water colour compound and it is high soluble in water. The main chemical element of borax is boron that is denoted as the symbol B in the periodic table. The atomic number and atomic weight of boron are 5 and 10.81 amu respectively. Borax or also known as Sodium tetraborate is very useful to prevent fungus due to its antifungal properties.
FAQs
Q1. Is borax a base or an acid?
Ans. Borax is a type of weak base mostly used for buffer solutions and photographic developments. Boric acid gets subtracted from borax with the help of various strong acid reactions.
Q2. What is the pH level of borax?
Ans. Borax is also known as sodium tetraborate decahydrate that hydrolyses to boric acid and OH- ions when it is dissolved into the water. Therefore, its pH is about 9.13.
Q3. Is borax and baking soda the same?
Ans. Borax and baking soda are not the same. Borax is known as sodium tetraborate whereas baking soda is known as sodium bicarbonate. The pH of baking soda is 8.
Uses of Biogas
Introduction
Biogas is considered a greener fuel and its popularity has increased in preset times. It is made with the help of organic waste, which produces the required methane. However, when the waste is not managed with the proper process, it creates a major risk to the environment. The process of making the biogas helps to reduce those risk factors.
What is Biogas?
Biogas is a mixture of carbon dioxide and methane and is considered a renewable energy source. The process of making biogas requires the decomposition of all the organic waste. It can break the organic matter such as animal waste, and plant waste and includes food scraps. Biogas is used as fuel for cars and cooking gas and it can generate heat for household use, which can replace the traditional form of electricity.

Figure 1: Biogas Plant
The mixture of biogas, consists of one more gas along with carbon dioxide and methane, it is hydrogen sulphide. The main source of those gases is agricultural waste, municipal waste, plant material and green waste.
Process of making Biogas
Biogas is referred to as the most environmentally friendly and renewable source of gas. The main thing that is used to make biogas is organic waste. Biogas is produced when food waste or other animal waste goes through the process named anaerobic digestion.
In this process, microorganisms help to decompose the waste material and it produces methane. This renewable energy source is getting popularity because of the high requirement for energy. To fulfil the high demand more people are showing interest in the biogas plant to generate fuel.
Figure 2: Process of making biogas
The main thing that is required to make the biogas is cow dung; it is considered the main source. It consists of many bacteria that help the anaerobic decomposition of the organic waste. It is the reason biogas is referred to as gobar gas. Cow dung has methanobacterium, which helps to produce methane gas and it produces the manure that is essential in the decomposition of organic waste.
Biogas is produced in an anaerobic digester, where all the cow dung and organic waste treats together, it is known as a biogas plant. It is made with bricks and cement and making the biogas, it needs a few steps that should be followed accordingly.
Mixing tank
Inlet chamber
Digester
Outlet chamber
Overflow tank
Uses of Biogas
In biogas, a few gases can be found in a certain percentage. For example, methane can be 50 to 60 %, carbon dioxide can be 25 to 30%, nitrogen can be present up to 9 %, hydrogen can be found at 1%, and hydrogen sulphide and oxygen can be found in 0.01 to 0.05% and 0.5%, respectively. Other than those gases, water vapour is also present in the biogas, and the amount depends on the temperature and moisture level of the atmosphere.
Biogas has many uses such as-
In rural areas, it is used as cooking gas
It can be used in the production of electricity
It helps heat the water or room
Biogas is the perfect replacement for the compressed natural gas that is used in vehicles.
It can be used in CHP plants
In transportation, it can be used, for example in Sweden it is used in Amanda Biogas Train.
In the process of making the biogas, manure is produced which is considered a useful by-product.
In many states, biogas is used to light up streetlights.
It is useful in hydrogen fuel cells.
It can be used as fertilizer too.
Advantages and Disadvantages of Biogas
There are many advantages and disadvantages of using biogas, such as-
The most important fact about biogas is that it is a renewable source.
It helps to get rid of the waste material and in that way, it contributes to the environment.
It reduces the pollution of soil and water.
It helps in the reduction of methane emitted to the atmosphere, which positively affects climate change and leaves an impact on the environment.
Along with many advantages, there are some disadvantages too −
As the process of making biogas is a biological process, it cannot be controlled completely.
The process cannot be done anywhere in this world, because it requires warm weather.
Conclusion
Biogas is the best source of energy, which can replace natural gas with the slightest cleaning. It leaves a lower impact on the environment in comparison with any other gas. On one side, it does not affect the environment and on the other side, it is carbon-neutral. The vast list of usage is meaning it more popular day by day.
FAQs
Q1. What is the main advantage of biogas?
Ans. Biogas has many advantages, but the most important among them is that it is eco-friendly. It also helps in preventing soil and water pollution. It is produced by using simple and low-cost technology.
Q2. Is biogas can be used in cooking?
Ans. Biogas can be used in cooking just like other fuel gas. Those biogases, which are produced in the household-level biogas reactors, are perfect for cooking purposes.
Q3. Is biogas a perfect replacement for natural gas?
Ans. Biogas can be more helpful just with a minor clean-up process. It can be used for generating electricity and heat energy and in this way it can be considered a helpful replacement for natural gas.
Q4. Why biogas is good for the environment?
Ans. Biogas can be produced in the simplest process, with the help of organic matter such as food or animal waste. They go through a breakdown process by microorganisms and this happens in the absence of oxygen, which is called anaerobic digestion. Because of that biogas is considered environment friendly.
Uses Of Benzene
Introduction
Uses of benzene are mostly in tire and paper industries. The organic compound benzene was first discovered in the 19th century. The person who was able to find this organic compound is a scientist from England. This scientist is Michael Faraday who illuminated a gas to produce benzene. In this case, the process of derivation followed by Michael Faraday was to take a liquid apart from a condensed state of oil and gas.
Structure and formula of benzene
The structure of benzene is consisted of cyclic unsaturated hydrocarbon. The ratio of carbon and hydrogen present in the compound lacks a balance. All the six carbons that are found in a molecule are closely attached to the hydrogen atom. The entire arrangement is found in the form of a few rings that go around the molecule of benzene. These are the basic attributes of the structure that are found in an organic compound of benzene. The formula of benzene is known as C6H6 where, six carbons are attached over a single hydrogen atom.
Physical and Chemical Properties of Benzene
Benzene melts is an organic compound that does not have any colour.
The compound is found usually in the form of a liquid.
The melting point of benzene is 5. 5°C and the point where it boils is 80.1°C.
Benzene holds a certain amount of solubility in solvents found in its organic state.
The organic compound has an odour that is very aromatic.
The result of combusting benzene is the formation of flame-covered soot.
This organic compound is electrophilic by nature.
The reaction of fluorine with benzene is very aggressive when the catalyst is absent.
Benzene goes through a process of halogenation in the presence of Lewis acids.
Acyl benzene is formed when acyl halides react with it when Lewis acids are present.
Preparation of Benzene

Figure 1: Preparation of zinc from phenol
The preparation of benzene is done by making sulphuric acid go through the process of hydrolysis. In this case, a large amount of steam which is very high in temperature is put inside the acid to create benzene. Benzene is produced from ethane where the latter needs to go through a very hot tube whose temperature is 873K. The entire process is known as cyclic polymerization which produces benzene. The third method of preparing benzene requires heating phenol with metal called zinc. Dust of zinc gets some hot phenol to start the reaction. Thus the result is the formation of phenoxide ions that is finally converted into benzene.
Uses of Benzene
The manufacturing of tires and rubber needs a large amount of benzene. The production of these materials requires some steps to complete the production. Benzene is used most of the time in these particular steps to make quality tires and rubbers.
The industry where papers and journals are printed is automated due to the presence of dynamic printing machines. Therefore, the machine must be cleaned quite often to increase its efficiency. This cleaning agent contains benzene that the machines to stay in good shape.

Figure 2: Uses of benzene
Flammable products used to obtain energy like gasoline; fuels and kerosene are rich in benzene. There are many by-products of these substances for example lubricants used on metal joints have a considerable amount of benzene.
The use of benzene is very common in the industry where materials of plastic are manufactured. These are considered one of the ideal substances used in the production of Styrofoam and styrene.
This organic compound has its fair share of uses in the chemical industry. This is the reason behind the fact that benzene is a necessary component used in the production of various chemical fertilisers and dyes.
Facts about Benzene
There is no limit to the use of benzene in its pure form. Even after that, this compound helps in the production of various other chemicals.
Benzene has a nice aroma that was used as a lotion after shaving the beard and moustache.
The use of benzene is very significant as it made ranks in the top 20 chemicals used all the time in a year.
Benzene is not very safe for human beings but these are present inside all household items.
The total weight of benzene is more than the surrounding air hence stay suspended at a very low altitude.
Conclusion
The tutorial explains the uses of benzene in modern society made by human beings. The formation of this organic compound has various ways that imply the traits of inorganic minerals. These compounds have physical properties that show their ability to synthesise themselves as some product. The uses range from cosmetic products to industries manufacturing canned and processed food items. There are a few facts that further explain its uses to all learners.
FAQs
Q1. What are the bonds that are found in the structure of benzene?
Ans. The entire structure of benzene is bonded by a structure of hydrocarbons that are attached to one another by a covalent bond. The structure takes the shape of a ring and remains a stable bond for an extended period of time.
Q2. What is the factor that influences the stability of benzene?
Ans. There is a single factor that plays large to make this organic compound stable. The constituent is attached by covalent bonds and placed in the form of rings. Now the rings have electrons that are not local to a place. Lastly, the electrons are situated at a place that is above and beneath the rings. These are the ways the organic compound stays stable.
Q3. What makes benzene harmful to the body of a human being?
Ans. The organic compound of benzene is firstly carcinogenic as it induces the probability of getting cancer in the body. Secondly, the consumption of benzene prohibits the body from working the way it should.
Uses of Bauxite
Introduction
Uses of bauxite are described with proper applications in metallurgy industries. It was manufactured in the early decades of 19th century. This metal ore was named after a small town in the state of France. A chemist from the country, whose name was P. Berthier, first discovered this ore. Those are extracted from nature by using different methods and techniques.
Types of Bauxite
Two different classifications of bauxite are found to exist on the planet. The first type is known as the lateritic bauxites. On the other hand, the second type is known as karst bauxite. Each of these bauxites has different categories for specific purposes of usages. The lateritic bauxites are found in a specific profile of soil which is rich in laterite. The raw materials extracted from the source of bauxite mines are stacked with deposits of aluminosilicate. Hence, aluminium is extracted from bauxites using the process of hydration.
The second type of this element is known as karst bauxite, which lies on rocks in its carbonate form. The formation of this type of bauxite occurs on the karst soil which is rich in hematite. In this structure, karst bauxite has a heavy deposition of aluminium oxides. These types of bauxites have the same use to provide a certain amount of aluminium just like the lateritic bauxites.
Properties of Bauxite
The properties of bauxite are listed down under this section. These hold a large variation in their physical attributes. Thus, these are listed down under this section −
The colour of this bauxite ranges from reddish brown to white and grey at times.
The ore of bauxite is very soft in nature as the scale of Mohs ranges between the scales of 1 to 3.
The structure of all the bauxite ore is usually found in the pisolitic state.
These ores of bauxite also lack a low specific gravity as it becomes a variable between the scales of 2.0 to 2.5.
The aspects of lustre observed in the ores of bauxite are usually dull and have an earthy attribute.
The diaphaneity of bauxite ores is usually opaque.
There is no cleavage formed between the ores of bauxite.
These are some of the properties of bauxite found in its natural state when these are extracted from the mines.
Extraction of Bauxite
The process of extraction of bauxite ores is a very simple endeavour as it requires open-cast mining. In this process, the land where very large reserve of bauxite is believed to present is cleared off by plucking all the vegetation along with seeds collected. After mining, the soil layer can be retained and the vegetation as well as the seed can be placed inside the soil again for plantation of species one more time. The process of mining starts with the removal and storage of the topsoil to reach the deposit.
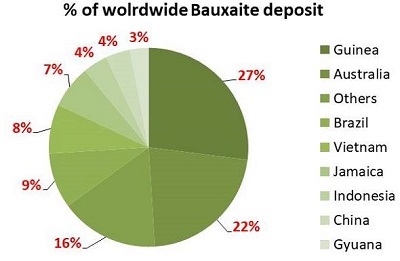
Figure 1: Percentage of worldwide bauxite deposits
There is a huge of rocks and soil known as overburden. This hard layer of rocks is drilled and broken into pieces, which is convenience to the transportation of these bauxite ores. These are transported to the refinery for the extraction of aluminium or for the purpose of washing any dirt from the ores.
Uses of Bauxite
In order to derive aluminium, the metallurgy industry uses bauxite ores.
The production of different parts of a car and utensils are made up using bauxites.
This type of ore is used in the production of abrasives and cement.
Figure 2: Uses of Bauxite
Bauxite gives aluminium is widely used in the production of tools found in every household.
The use of bauxite is very important in the electronic industry as aluminium is a major constituent in the production of antennas for radios.
Lateritic bauxites help in the construction of roads and components used for the purpose of building houses.
Facts about Bauxite
The facts about bauxite are as follows −
The places where the price of electricity is affordable to support the production of aluminium.
The form of sintered bauxite is found in the process of hydraulic fracturing.
The beads found in the shape of spheres are very hard and durable by nature.
The Bayer’s process is used to filter every ounce of aluminium s found inside the ore of bauxite.
Other bauxites are found on the planet works in the same manner. The only difference is that it should be obtained at a very high cost compared to bauxite ores.
Conclusion
The tutorial explains the process by which the reservoirs of bauxite are mined for the production of different items. The tutorial gives brief idea about the methods used to extract the ores of bauxite. There are some properties of the bauxite ore that helps to make different types of objects on the basis of these properties. The images are here to give a brief overview of the tutorial to all the learners.
FAQs
Q1. Who is the person to find the metal ore of bauxite?
Ans. The person who was the first to find the metal ore of bauxite was P. Berthier. This man was a chemist from France who named the mineral ore bauxite.
Q2. What is used to preserve the mining reserve of bauxite?
Ans. The unique method commonly known as the Bayer’s Process is the best way to preserve bauxite. This is the method that is used to protect the large reserves of bauxite.
Q3. What is the impact on the environment due to mining bauxite ores?
Ans. There are severe impacts on the environment due to the mining of bauxite ores. Deforestation is the first problem as all kinds of mining demand cutting down trees. The next includes massive dust pollution and a vast landscape of wasteland that is unfit for vegetation and cultivation.
Uses of Ascorbic Acid
Introduction
Ascorbic acid is known with common name of Vitamin C and an essential chemical element for humans. It is normally found in vegetables as well as fruits and the exposure of the substance to the sun turns into darker. The physical properties consist of a white or slightly yellow crystal or powder having an acidic taste.
Overview of Ascorbic Acid
The ascorbic acid mainly belongs to the family of monosaccharides and bears the chemical formula C6H8O6. It is also named vitamin C and known as the most important vitamin for plants as well as animals. The addition of this vitamin into the diet is most important, as human body is not able to synthesize this vitamin.
Figure 1: Ascorbic acid
Jü, Public domain, via Wikimedia Commons
The other name of this acid is Vitamin C or L- ascorbic acid. The presence of this vitamin has been seen in citrus fruits, broccoli, strawberries, kiwifruit, raw bell pepper, as well as in Brussels sprouts. This organic compound is considered as an oxidant and used as a reducing agent. This is soluble in water and also found in tomatoes, potatoes, as well as leafy vegetables.
This acid is widely used in preventing as well as in treatment of common cough and cold. It is applied to the skin for protection from the pollution as well as the sun. The people, who suffer from the disease of Alzheimer's, as well as depression, also take vitamin C for curing their problems.
Applications of Ascorbic Acid
Ascorbic acid is called one of the most important vitamins and human beings need this vitamin very much. Therefore, the most common use is noticed in the field of medicine. The uses are described below −
Different Health Benefits
It is called an antioxidant and because, it helps in recovery from that damage. It helps in healing the damage by the radiation or by inhalation of tobacco smoke.
In Personal Care
Ascorbic acid acts as an antioxidant in the products of personal care as well as cosmetics such as, makeup products. This acid can observe also in the products of hair as well as skincare.
Beverages and Foods
These kinds of acid are also used in acidity regulators, preservatives, nutrition supplements, and colour fixatives in the field of beverages and food.
Animal Fodder as well as Agriculture
One of the essential applications has been seen is the field of agriculture or animals, as well as in poultry food. It is used as a supplement to nutrition in those fields.
These are the benefits as well as uses of this type of acid and also helpful for curing the problem of anaemia. The intake of this acid in the form of medicines helps in reducing the deficiency of vitamin C and help in maintaining the irregular heartbeat. Some other benefits are: it can help to get rid of common cold, reducing pain, different infections, high level of blood pressure, and as well as cholesterol.
Negative Effects of Ascorbic Acid
The absorption of ascorbic acid sometimes makes some side effects on humans and some symptoms are: hives, allergies, swelling of lips, tongue, face, throat, and as well as difficulty in breathing. Some side effects are as follows −
Blood in the urine.
Severe pain in the lower back.
Fever
Chills
Painful urination.
Frequent urge to urinate.
Loss of weight.
Pain in the stomach.
Joint pain
Feeling of tiredness.
Heartburn
Upset stomach
Nausea
Diarrhoea
Cramps in the stomach.
Ascorbic acid Properties
Ascorbic acid consists of different properties and these are described below −
| Chemical formula of Ascorbic acid | |
| Weight of the molecule | 176.124 g/mol |
| Density | 1.65 g/cm3 |
| Appearance | Light yellow solid |
| Melting point | 190-192°C |
Table 1: Properties of ascorbic acid
Conclusion
The tutorial provides about ascorbic acid with the empirical formula of . It is seen that when the acid remains in the dry state becomes stable. It has been seen that when the acid comes in contact of air remain stable and unreacted but, when it is dissolved into water forms solution, it oxidises quickly. The main applications of this acid are seen in the medical fields and it helps to deal with the deficiency vitamin C.
FAQs
Q1. What are the uses of ?
Ans. It is good for the bones as well as the connective tissues, vessels of blood, and for muscles. It also assists in the absorption of iron and important for red blood cell development. It is applied for treatment of deficiency of vitamin C as well as in the process of medication.
Q2. What is the nature of ascorbic acid?
Ans. This acid has a very high acidic nature and when it is consumed stomach should not be empty, it creates gastrointestinal side effects. It consists of a low level of pH and therefore, calcium ascorbate forms for reducing the adverse effect of epigastric.
Q3. What is the similarity between vitamin C and ascorbic acid?
Ans. It is seen that ascorbic acid does not act as a vitamin when it lies in the form of chemically isolated. This acid is called one of the clones of the living complex components called vitamin C. Therefore, this acid is a crystalline, fractionated vitamin C isolate.
Q4. What foods contain ascorbic acid?
Ans. Some foods are rich with ascorbic acid and these are cauliflower, broccoli, tomatoes, kiwi, yellow, and green pepper, kale, cantaloupe, papaya, sweet potatoes, orange juice, as well as strawberries.
Uses of Ammonia
Introduction
Ammonia is a gas that is made naturally in the body of humans as well as in nature. It is found in soil, water, and air, as well as in bacteria. Ammonia, as well as ammonium ions, is considered as the most important components for human body as it helps in metabolic processes.
What is ammonia?
Ammonia is nothing but a gas that has no colour but a pungent smell. It is able to dissolve into water, furnishes strong alkaline solution. The solution of ammonia is mainly used as a cleaning fluid.
Figure 1: Molecular Structure of Ammonia
Ammonia bears the formula of NH3 and this compound occurs naturally and make through the activities of humans. This compound is called one of the most important sources of nitrogen. The animals as well as the plants require this nitrogen produced by ammonia. Bacteria present in the intestine mainly produce ammonia.
Many people may know about the odour of ammonia because this compound has been using in smelling salts, industrial cleaners, as well as household works. The liquid ammonia when exposed to air turns into gas, which can be used directly into the soil for farming.
Properties of ammonia
The physical, as well as chemical properties of ammonia are described below −
Physical Properties
| Chemical formula of ammonia | NH3 |
| Appearance | A gas having no colour |
| Odour | Strong and pungent odour |
| The point where it melts | -77.73°C or -107.91F or 195.42 K |
| The point where it boiled | 33.34°C or -28.01F or 239.81 K |
| Density | 0.86 kg/m3 |
Table 1: Physical properties of ammonia
Chemical Properties
The chemical properties are as follows −
This compound is highly soluble in water and the aqueous solution of NH3 is called a weak base until the formation of OH- takes place.
The reaction of ammonia with acid mainly forms ammonium salts.
Uses of Ammonia
Ammonia is basically a gas naturally found in the environment such as, in soil, water, as well as in air. This compound is called the essential building-block chemical in manufacturing different products. People use various things in their everyday life and which are made up using ammonia. The usefulness of ammonia are as follows −
In the field of the industry
NH3 is mostly used in different industries and as a neutralizer as well as a stabilizer. This is called the source of nitrogen that helps in performing different functions. The main application of this compound is seen in the treatment of wastewater, rubber, beverage, leather, as well as food industries.
Another use of this compound has been seen in cold storage, in the system of refrigeration, and in the creation of pharmaceutical products. The industries of cosmetics, as well as the printing industries, also use this compound and along with its use is in the process of fermentation.
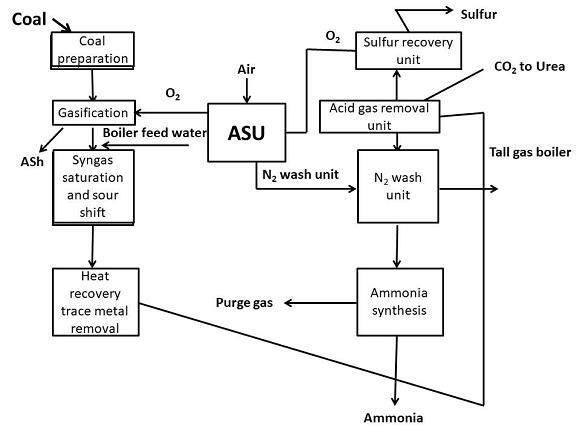
Figure 2: Ammonia synthesis from coal
The Products of the Household
The various cleaning products in the household are manufactured using ammonia and known as the most essential ingredient of those products. This compound is mostly used as an agent which helps in cleaning or removing stains on sinks, mirrors, tubs, as well as windows. Ammonia is applied as fuel and it is also used in the preparation of antiseptic or an antimicrobial agent.
In Agriculture
The industries related to agriculture mostly use the compound. Ammonia is known as a very rich source of nitrogen and used in fertilizers. This fertilizer helps in the production of food and the application has been seen in making liquid fertilizer solutions. It is consist of some compounds such as, nitrate of ammonia, ammonium salts, urea, as well as others. This is called an antifungal agent used in different foods and helps in the preservation.
The Manufacturing of Different Compounds
NH3 helps in manufacturing different compounds such as, ammonium carbonate, nitric acid, hydrogen cyanide, amino acids, urea, phenol, and many more.
Treatment of Metal
Dissociated form of ammonia is commonly used in different operations such as, nitriding, sintering, carbo nitriding, furnace brazing, atomic hydrogen welding, bright annealing, and many other operations.
In Mining as well as Petroleum
The industries of petroleum, as well as, the mining industries, have been used ammonia for counterbalancing acid constituents of oil and known as crude form. This matter helps in keeping the equipment free from corrosion. This also helps in the industries of mining in extracting different metals.
Conclusion
Ammonia is consisting of the formula NH3 and a colourless gas. This has a pungent odour found naturally. The human body produces ammonia during digesting of foods and consist of ammonia and amino acids. After that, it converts ammonia into urea, which plays a significant role in manufacturing various products.
FAQs
Q1. What are the harmful effects of ammonia?
Ans. Ammonia is known as a natural compound as found naturally everywhere. This compound also has harmful effects when it is consumed in a high amount and they are coughing, irritation in the eyes, problem in the throat and lungs, and skin irritation.
Q2. What is the reason for ammonia production?
Ans. Ammonia is mainly produced through the distillation of animal waste products as well as some vegetable wastes that are rich with nitrogen. It can be obtained naturally from the environment and through the process of Haber-Bosch.
Uses of Amines
Introduction
Uses of amines refers to its applicability in textile industries, medicinal industries etc. Ammonia is the main source of amines and one of the known organic compounds. Various products are derived from ammonia and amine is one of them. Amine, as an organic compound acts as starting agent in the syntheses of other organic and inorganic products.
What are amines?
Amine is a type of chemical compound sourced from NH3 or ammonia. It belongs to the organic nitrogen compounds in its functional group containing a lone pair of nitrogen atoms. Alkaloids are consists of various degree of amines in its structure occurs naturally. These compounds are found in some plants including histamine, epinephrine, norepinephrine, and dopamine.
The number of carbon bonds with nitrogen atoms classifies amines into different degree of molecules such as, tertiary, secondary, and primary. The compound acts as an ammonia derivative fulfils various purposes related to industrial sectors. Amine is used as a core product and also as by-product in pharmaceutical and agrochemicals industries.
Type of Amines
Amines are classified into three types based on its degree of replacement of hydrogen atom from amine structure. These are: primary amines, secondary amines, and tertiary amines.
Primary Amines
Primary amines are produced by the replacement of one hydrogen atom from the organic compound of ammonia. An aromatic group or alkyl does this task. Some instances of primary alkyl amines include methyl amine and amino acids.
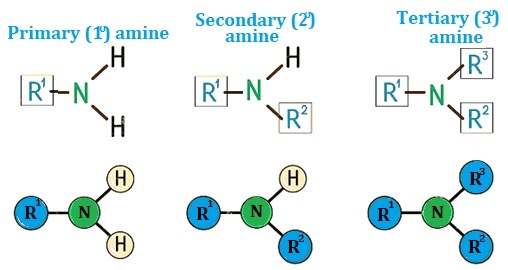
Figure 1: Classification of Amines
Secondary Amines
If Aryl, alkyl or both organic substitutes two hydrogen atoms of organic compound of ammonia results in secondary amines.
Tertiary amines
When three organic substitutes are attached with ammonia based organic compounds then it is designated as tertiary amines.
Composition of Amines
Amines are organic compounds and related to the functional organic nitrogen group. It consists of a lone pair of nitrogen atoms. Hydrogen bonds are formed between amine groups and water molecules and results in water solubility along with an increasing its boiling points. The compounds which belong to a carbonyl group attached with amine group are designated as amino acid compound. Amino acid structure is expressed as R-CO-NR’R, here, amine group is in tertiary degree by replacing both hydrogen atoms with alkyl or aryl groups.
The main structure of this compound is responsible for its wide usage. Aromatic amine structures are formed by the attachment of amine groups to the benzene ring in single or multiple number results in alkaline characteristic of the benzene ring towards reduction reaction. When it comes to electron donation, the aromatic amine compounds as compared to aliphatic amines are less reactive due to delocalization of lone pair of Nitrogen atom towards benzene ring. Amine structure is composed of trivalent nitrogen atoms and an undistributed electron pair.
Primary amines are formed with one hydrogen atom replacement out of three by an aromatic element or alkyl group. The replaced hydrogen atom count is two then appears secondary amines. An organic substitute replaces all three hydrogen atoms produces tertiary amines. For saturated or unsaturated ring like structure of amines only can be classified into tertiary and secondary degrees.
Physical Characteristics of Amines
Properties of amines and its structure contribute to its usage in various field. Its physical properties are as follows −
The boiling point of the compounds is high and soluble in water as it consists of hydrogen atoms in amine groups. The solubility decreases in water with increasing degree of the amine compounds that results in an increase of carbon atoms in the structure.
Variations of the number of carbon atoms in amine compounds differs its state of existence. Those compounds are in a typical gaseous state with less number of carbon atoms. It appears with a fishy odour. The amines are in liquid state with the content of three carbon atoms in the amine structures. The amines appear as solid substances when the carbon atom count is more than three.
The amines mostly have no colour. Atmospheric oxidation can help amines to obtain some colours.
Chemical Characteristics of Amines
Some important chemical characteristics of amines are described below −
Amines are basic. An increase of alkyl groups increases basicity of the amines.
Amines participate in various chemical reactions. The processes include acylation, alkylation, carbylamine reactions, and electrophilic substitution.
Amines, as dedicated sources of ammonia perform reactions with aryl sulfonyl chloride, nitrous acid and produces an oily substance in yellowish colour.
Applications of amines
Amines are used for various daily needs and these have pharmaceutical uses.
Amines in Daily Life
Material dyes are prepared using amines as core material.
Amiens is used for treating other gases. Combustion of gases makes free of CO2 by using amines.
In garments and textile industries, azo dyes are critically required to treat substances like, nylon and leather. Amines are used to prepare azo dyes.
Lubricating oils and boilers in chemical processing industries can be prevented from corrosion by using amines.
Amines are used to increase solubility of herbicides. These are also known as emulsifiers.
It is also used for photograph development.
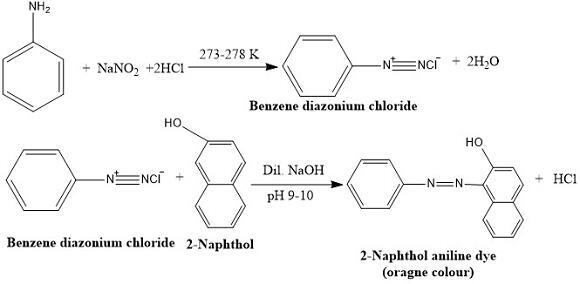
Figure 2: Amines are used for dye preparation in commercial scale
Amines in Pharmacy
Demerol and morphine are two known painkillers contains amine as functional group with significant medicinal applicabilities. Amines have frequent usage in these medicines.
Novocaine is an anaesthetic drug. It has a high dependency on amines.
Benadryl syrups have the use of antihistamine diphenhydramine. One of its solvents is Amines.
Neurotransmitter in the body requires stimulants like serotonin. Amines are also a good stimulant for the same purpose.
Amino acids in the body can regulate the levels of vitamins and they can be sourced by amines.
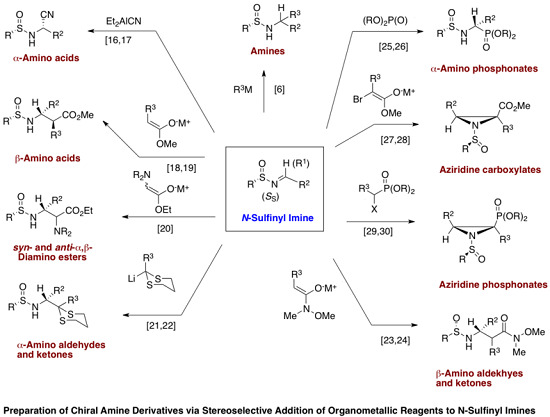
Figure 3: N-Sulfinyl Imines in Preparation of Chiral Amine Derivatives are used in medicinal drug production
Professorattemple, Sulfinimine Applications, CC BY-SA 3.0
Conclusion
Alkyl or aromatic groups replace hydrogen atoms from organic compound of ammonia and these organic compounds are classified as primary, secondary, and tertiary amines. Amines are generally colourless and have a high boiling point. These compounds are used in chemical industries and pharmaceutical sectors.
FAQs
Q1. What is meant by charge of amine?
Ans. Three substituents, one nitrogen atom, at least two hydrogen atoms, and a lone electron pair collectively considered as an amine functional group. The lone pair acts as electron donating agent towards any electron deficient group or solution. Hence, amines are electron rich and considered as negatively charged due to presence of electron pair. The binding of nitrogen occurs with 4 substituent and the nitrogen atom remains with a positive charge.
Q2. What is the functional group of amine?
Ans. Amines are derived from the processing of ammonia. Hence, it is called as organic compound of ammonia. An aryl p or alkyl group substitutes hydrogen atoms to produce amine. Therefore, primary amine consists of functional group of –NH2, secondary amines consists of functional group of –NHR, and tertiary amines exist with functional group of –NR3.
Q3. What are the factors that affect the basicity of amines?
Ans. Presence of electron-donating group in the same organic compound along with amine group increases basicity of amines, whereas, in presence of electron withdrawing group the organic compound with amine group reduces its basicity.
Uses Of Alloys
Introduction
Uses of alloys are very much significant in human life. All substances that exist are formed primarily by elements. As long as the use of metal elements has increased, its alloys become equally useful and the count of alloys is increasing day by day.
Meaning of alloy
Alloy can be defined as a mixture combined with two or more elements. Elements with distinct materials are used to prepare alloy. It can be composed using a couple of metal substances or by combining a metal with a non-metal. These mixtures are prepared through several steps of melting, pouring, solidification, and many more. In industries, alloys are prepared in complex procedures.

Figure 1: Common alloys are in combination of various pure metals
The properties of an alloy generally are superior as compared to the properties of individual metals from where it has been derived. Magnalium, Bronze, Duralumin, and Brass are some examples of alloys. Alloys offer better strength and durability than their original metals or non-metals.
Characteristics of alloy
Alloys present different properties than the original substances are composed of.
Important characteristics commonly found in alloys are mentioned below −
Toughness
It is one of the very important properties of alloys responsible for their initial employment for usage. Steel is the alloy where the participant elements are iron and carbon. The alloy offers better properties than iron after the addition of carbon.
Ductility and Malleability
Some metals are changed into sheets by transformation and this property is called malleability. Most metals can be shaped into thread or wire form using this property called ductility. Both of these properties are well utilized in gold alloy. Pure gold is easily deformable and very soft. If it is mixed with a foreign element, the deformation can be avoided and the gold alloy can be used to give any shape.
Magnetizability Customization
Magnetizability of an alloy can be altered by tweaking the composing elements. Magnetic induction of Steel can be controlled by including or excluding manganese.
Non-corrosive
Element decay occurs due to the unwanted and natural process of corrosion. Making Alloy helping things to become non-corrosive. Bronze is less corrosive than copper.
Advantages of Alloy
An alloy is more advantageous than its pure elements. Some common alloys with its advantages are discussed below −
The melting points of pure metals are generally very high. Making alloy helps them to melt in reduced temperatures.
The tensile strength of an alloy is more than its parent elements. Hardness of metal can be increased by adding other non-metal or metal to it.
Metals have extreme susceptibility to weather and chemical attacks. Alloys offer better inertness than pure metals and also corrosion resistant.
The contraction of metals occurs during solidification. The preparation of an alloy from its pure metal, offers expansion during solidification and better castings are obtained as a result.
A special advantage of creating alloy is a colour change. Alloys are created to present a different colour from parent metals.
Compositions of Some Alloys
Some alloys and their components are mentioned below −
| Name of alloy | Components |
|---|---|
| Bronze | 88% of copper and 12% of tin |
| Brass | 15 to 40% of zinc and 60 to 85% of copper |
| Steel | Iron and few amounts (1%) of carbon |
| Alnico | Aluminium, cobalt, and nickel |
| Cast iron | 2 to 4% of carbon and 96 to 98% of iron |
| Solder | Lead and tin |
| Sterling silver | 92.5% of silver and 7.5% of copper or other metal |
| Nichrome | Chromium, nickel, and iron |
| Magnalium | Magnesium and aluminium |
| Duralumin | Copper and aluminium |
Table 1: Different alloys and composition of possible parent metal
Some common usages of alloys in everyday life are described below −
Construction of bridges, railways, airports, and roads has the use of steel. Many items used for household needs are also made with steel.
Bronze is used to make medals, musical instruments, and mini sculptures.
Brass alloy is used to make door handles, locks, doorknobs, zippers, electrical appliances. The alloy is also used for making gifting items and decorations.
Alnico is used for creating permanent magnets.
Many surgical instruments, musical instruments, jewellery pieces and cutleries are made from sterling silver.

Figure 2: Elgiloy alloy parts
phynox alloy, Elgiloy alloy parts .2018JPG, CC BY-SA 4.0
Permanent joining is required for electrical components and solder is used for this purpose.
Aluminium alloys like, magnalium is known for its lightweight. These are used to make aircraft structure and parts.
A mercury alloy called amalgam is used for various medical purposes. Tooth cavities are filled with this alloy.
Gold alloys like rose gold is used to create jewellery.
In aerospace industries, extensive usage of an alloy called titanium occurs due to superplastic behaviour and strength of high-temperature tolerance.
Conclusion
An alloy is defined as a mixture of two or more metal or non-metal elements offering different or altered properties than pure metal or non-metal elements. There are some alloys of metal and non-metal elements, which offer better durability than parent substances. Alloys are made for getting enough hardness and can be used in daily life in huge amounts due to their inertness. Alloys of different metals offer features like non-corrosiveness, colour change, low melting point and many other features.
FAQs
Q1. What difference do you find between metal and non-metal?
Ans. Metals are generally solid and shiny substances with high amounts of thermal and electrical conductivity. The melting and boiling points are high in metals. Non-metals are generally soft substances found in liquid, gaseous, or solid form.
Q2. Why magnalium is used?
Ans. Magnalium is an alloy made up of 5 wt. % magnesium and 95 wt. %aluminium. The alloy shows better temperature resistance and durability than aluminium. It is widely used in aerospace industries for constructing aircraft and aircraft parts.
Q3. What are the additional benefits of alloy or metals?
Ans. An alloy made of metals and non-metal offers better strength and durability. There are many other benefits including inertness, non-corrosiveness, ductility, and malleability.
Physical and Chemical Properties of Alcohols
Introduction
The organic compounds that form a series that consists of the same functional groups but have variations in their group are determined as the homologous series. In this homologous series, various successive compounds can be seen including alcohol, phenol, ether, and so on. The alcohols have more than one functional group that is quite similar to the functional groups of phenols and ether.
Alcohol
The organic compound in which the atom of hydrogen or the aliphatic carbon gets replaced by the hydroxyl group is referred to as the alcohols. The compound can be identified by its sweet smell. Alcohols can be of 4 types: ethyl, denatured, isopropyl and rubbing alcohols. In all these, alcohol types, ethyl alcohol are widely used which can also be referred to as grain alcohol or ethanol. Ethyl alcohol is widely used for the preparation of beverages like wine, beer, and other liquors. Alcoholic beverages are generally made with yeast and fermented sugar.
The major simple forms of alcohol include methanol , ethanol , propanol and butanol .
Structure of Alcohol
The structure of alcohol is generally attributed due to the presence of the hydroxyl group. The atom of carbon that is present in the molecule of alcohol the atom of carbon remains bonded to the oxygen atom of the hydroxyl group with the help of a sigma bond. The sigma bond is usually denoted by the symbol σ.
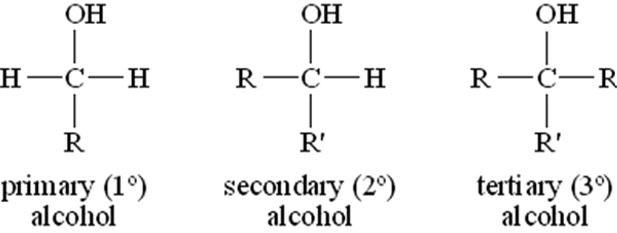
Figure 1: Structure of Alcohols
The bond in the alcohol molecule is created because the overlap of an sp3 hybridized orbital of carbon takes place with an hybridized orbital of oxygen. Repulsion takes place between the oxygen electron pairs that remain in an unshared state.
Types of Alcohols
Various types of alcohols can be used for several medical, industrial, and technical purposes as discussed below.
Ethanol
Ethanol is also referred to as the intoxicating component that is utilised in the preparation of various alcoholic beverages including, distilled spirits, wine, and beer. The molecule formula of ethanol can be written as . It has a density of 789 kg/m3 and a molar mass of 46.07 gm/mol. The properties of ethanol highlight that, it is a courless liquid having a burning taste and sweet smell.
Figure 2: Structure of Ethanol
Propanol
Propanol can also be referred to as Isopropyl alcohol that is prepared through indirect hydration of propylene. Propanol or Isopropyl alcohol can be used as rubbing alcohol and solvent in various cosmetic and medical industries that be applied to the skin. Propanol is more toxic than ethanol or ethyl alcohol.

Glycerol
Glycerol also determined as glycerine, is the sweet syrup that has 3 alcohol hydroxyl groups. Propane-1,2,3-triol is the systematic name of glycerol which is generated through sugar and molasses fermentation. A large amount of glycerol is required in order to prepare glyceryl trinitrate (nitroglycerin).
Properties of Alcohols
The common chemical and physical properties of alcohols are stated below.
Chemical Properties
Reaction with phosphorus halide − Alcohols get reacts with Phosphorus halide and prepare haloalkanes. The reaction can be written as: .
Reaction with Grignard reagent − Alcohols get react with Grignard reagents in order to produce hydrocarbons. The reaction can be written as: .
Reaction with thionyl chloride − In pyridine, alcohols are treated with thionyl chloride to produce chloroalkanes. The reaction can be stated as: .
Physical properties
Higher alcohols get solidified at room temperature whereas lower alcohols remain in the liquid state.
Alcohols have a higher boiling point than haloalkanes that remain associated with intermolecular hydrogen bonding.
The boiling point of the alcohol compound decreases due to decrease in the surface area.
Solubility of alcohols in water reduces with the increase in their molecular mass and due to a reduction in the extent of intermolecular hydrogen bonding.
Applications of Alcohols
Some common applications or uses of alcohol are stated below.
Alcohol like Ethanol and methanol can be used as fuel to operate automobiles.
Consumption of alcoholic beverages can help in lowering the blood glucose level and act as a temporary pain reliever for humans.
Ethanol is also referred to as a type of alcohol widely used in several medical sectors as an intoxicating or anaesthetic agent.
One of the vital industrial chemicals is ethanol that is generally used as a solvent in order to synthesize other organic chemicals. It is also used as an additive to gasoline which is the component of refined petroleum and is used in automobiles and jets.
Conclusion
The compound that is derived when 1 atom of hydrogen gets replaced by a hydroxyl group within an aliphatic hydrocarbon is referred to as alcohols. Alcohol can be used for various industrial and medicinal purposes. In food and beverage industries the alcohols used in the preparation of beverages like wine, beer, whiskey, and other liquors occur widely after adding a little amount of yeast and fermented sugar in it.
FAQs
Q1. What is ethanol?
Ans. Ethanol is a chemical compound, that can be also determined as the ethyl alcohol, alcohol, or grain alcohol, a major member of the alcohol group. The molecular formula of ethanol can be written in the form of .
Q2. What the structure of alcohol compounds denotes?
Ans. The molecule of alcohol generally consists of two major parts including the alkyl group and hydroxyl group. The bond angle that can be seen in the case of an alcohol molecule is the bond angle, which remains a slightly tetrahedral angle (109°-28′).
Q3. What are properties of alcohols?
Ans. Alcohols generally react with hydrogen halide in order to prepare alkane halide. The reaction can be written as .The physical properties of alcohols denote that alcohol's acidic strength gets reduced with the electron number and with the increase of the donating carbon groups.
Allotropic Forms of Phosphorous
Introduction
The allotropes of phosphorus are found in different allotropic forms. The main allotropes of phosphorus include the white phosphorus, red phosphorus and black phosphorus. In addition to this, there is also the existence of violet phosphorus. The black phosphorus is black in colour while the white phosphorus bears yellowish colour and red phosphorus has red colour.
Allotropes of Phosphorus
Allotropy is called the science of occurrence of an element in multiple or more than one physical shape. Here allotropes refer to different types of physical shapes of the same element. It is seen that allotropes is the property of some chemical elements to exist in two or more different forms.
In nature, there is the presence of various allotropic forms of phosphorus. The important forms include −
White phosphorus
Black phosphorus
Red phosphorus
White Phosphorus
White phosphorus is also called tetra phosphorus and yellow phosphorus. It cannot be fetched naturally but it can be simulated from phosphate rocks. It has some features and they are as follows:
Physical properties
Some physical properties of white phosphorus are described below
It looks like a waxy solid because it is translucent.
It contains a garlic-like odour.
White phosphorus is known as a polar compound and the reason for this is that it is soluble in carbon dioxide
The molecular weight is 30.97g/mol.
It is corrosive and highly toxic in nature.
Chemical properties
The chemical properties of this phosphorus include
It results in metal phosphide when reacted with a metal.
It reacts with the oxygen present in the air and catches fire, and due to this reason it is stored underwater
It is seen that when this phosphorus is heated at 573K in an inert atmosphere for a few days, it creates red phosphorus.
Structure
The white phosphorus is made of four atoms and all the atoms are connected in a covalent bond. The structure of the white phosphorous looks like a ring and it creates an angle bond of 60°.
Red Phosphorus
Red phosphorus is another kind of allotrope of phosphorus and it can be got by heating the white phosphorus. It can be gotten through heating at a high temperature of around 573 K in an inert atmosphere for several days. It consists of some properties and has a specific structure and they are described below.
Physical properties
Some physical properties of red phosphorus as follows
It isodourless phosphorus.
Red phosphorus is non-toxic in nature.
It bears the melting point is 860 K
The molecular weight is 30.97 g/ mol.
It does not glow in the dark.
The name suggests that it is deep red in colour.
Chemical properties
Some chemical properties of red phosphorus include
This phosphorus reacts with oxygen at 565 K and results in phosphorus pentoxide.
It also reacts with sulfur and results in sulphides.
Structure
It is seen that red phosphorus contains the structure the same as black phosphorus and it is polymeric in nature.
Black Phosphorus
Black phosphorus is caused by heating red phosphorus at 416°C. The red phosphorus is converted into black phosphorus when it is heated in a sealed tube and at the right temperature. It includes some characteristics and they are as follows −
Physical properties − Some physical properties of black phosphorus are
It is black in colour.
The melting point is 416°C.
The specific gravity is 2.69.
Molecular weight is 30.97.
It exists in both crystalline and amorphous forms.

Figure 1: Physical properties of black phosphorus
Chemical properties − the chemical properties are
It is called the most stable allotrope among the allotrope of phosphorus.
It is the least reactive allotrope.
Structure − The structure of this phosphorus is zig-zag lines of P - P bonds. It mainly looks like a honeycomb and that is the reason it is called a honeycomb structure. The bond angles form 99° and here the bond length is 218 Pm.
Difference between Black, White, and Red phosphorus
| Serial No. | Basis | Black phosphorus | White phosphorus | Red phosphorus |
|---|---|---|---|---|
1 | Colour | Black | Yellowish | Red |
2 | Stability | Most stable | Least stable | Moderate stable |
3 | Reactivity | Least reactive | Most reactive | Moderate reactive |
Table 1: Difference between black, white, and red phosphorus
Uses of phosphorus
Phosphorus is most important for forming lives and it forms a basic constituent in the animal and plant matter.
It is found present in blood, bones and the brain of all the animals and also, in living cells.
Some of the compounds are applied in the industries and the most essential of these chemicals are orthophosphoric acid and phosphatic composts.
Conclusion
This tutorial shows different types of allotropes of phosphorus and they bear different characteristics. All the red, black, and white phosphorus contain both physical and chemical properties, and a structure. They have specific melting points and specific colours having the molecular weight. It states that the molecular weight of black phosphorus, white phosphorus, and red phosphorus is 30.97 g/ mol.
FAQs
Q1. Can white phosphorus be stored in the water?
Ans. The white phosphorus is considered to be the most reactive form of phosphorus. It mainly reacts with oxygen present in the air and it catches fire. That is the reason it is stored in the water for protecting it from the air.
Q2. Which is the most reactive allotrope of phosphorus?
Ans. It is seen that white phosphorus is the most reactive allotrope of phosphorus. Although it is most reactive; however, it is the least stable among all the allotrope of phosphorus.
Q3. Which phosphorus is applied in matchstick?
Ans. The red phosphorus is applied in a little amount in the matches. Here the potassium chlorate is ignited by the heat from this and thus the match gets flamed.
Photochemical Smog
Introduction
Photochemical smog is also famous as summer smog and it is very harmful. It is produced when UV light originating from the sun interacts with the oxides of nitrogen. This smog is usually personified as a brown haze in the atmosphere. It is seen in the highly populated cities that are placed in relatively warm climates. It is most see-able during afternoons as well as mornings.
What is Smog?
The modern world is delivering a hundred tons of waste every hour. Humans are mainly spoiling the rivers, the sea, and even the forest. All of these factors lead to the pollution of air at the end of the day. This pollution is a very pathetic thing because it is polluting the environment which is the only source of oxygen.
These factors lead to breathing problems in those who are already suffering from bronchitis and asthma. Nowadays many metropolitan cities are facing the issue of smog. It is called the mixture of smoke and fog that gets stuck in the air during the winter season. It is very risky to health and it also disrupts the ground-level ozone, sulphur dioxide, and carbon monoxide.
It becomes harmful to the senior citizens and here the most important factor is that it is linked to the pollution of the cities and towns. The classic smog pollution mainly happens when there is an excess amount of coal burning. It also increases the amount of smoke and sulphur dioxide in the air.
The result of this factor is the thick clouds of smoke which are known as smog. Photochemical smog is also known as the Los Angeles Smog. This type of smog occurs in the urban areas that contain a large number of automobiles.
Formation of Photochemical Smog
Photochemical smog can be formed with a complex series of chemical reactions. The matter includes sunlight, oxides of nitrogen, and volatile organic compounds present in the atmosphere as a result of air pollution. All those reactions often result in the formation of ground-level ozone and certain airborne particles. It is also observed that the appearance of photochemical smog is closely related to the concentration of primary pollutants in the atmosphere.
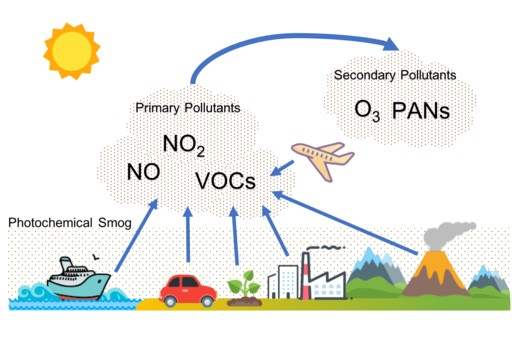
Figure 1: Photochemical Smog formation
(Image Attribution: Liweichao.vivian, CC BY-SA 4.0
The factor is also related to the concentration of secondary pollutants. Some common examples of primary pollutants those are responsible for photochemical smog is nitric oxide, nitrogen dioxide, and nitrous oxide and most VOCs. Some secondary pollutants are aldehydes, troposphere ozone, and peroxyacetyl nitrates. It is seen that during the peak traffic hours in the morning, large amounts of nitrogen oxides and volatile hydrocarbons are released into the atmosphere.
All the pollutants are traced to automobile emissions and industrial discharge. The hydroxyl groups in the atmosphere are mainly responsible for some of these hydrocarbon pollutants rapidly undergo oxidation. It results in the formation of peroxy radicals and these radicals go on to convert nitric oxide into nitrogen dioxide.
Composition of Photochemical Smog
Nitric oxide and nitrogen dioxide are emitted from the combustion of fossil fuels. It also reacts along with being naturally emitted from things like volcanoes and forest fires. The exposure to the ultraviolet radiation, goes through a complex series of reactions. The reaction happens with hydrocarbons to produce the components of photochemical smog.
It is called a mixture of ozone, nitric acid, aldehydes, peroxyacetyl nitrates, and other secondary pollutants. Here the , ozone and PANs are called photochemical oxidants. They are called so because they can react and oxidize certain compounds in the atmosphere or within a person's lungs that are not normally oxidized. It is seen that even small traces of these chemicals can affect the respiratory tract of humans and animals, and damage crops and trees.
What are the effects of Photochemical Smog?
Figure 2: Photochemical smog effects
Photochemical smog has different negative effects on the environment as well as human beings. It contained some chemicals and they combination with hydrocarbons form molecules which cause eye irritation.
Here, the atmospheric radicals interfere with the nitrogen cycle and stop ground-level ozone from being eliminated. The ground-level ozone can prove to be extremely toxic to human beings. Other negative effects are decreased vision and shortness of breath.
Conclusion
Smog is mainly called a by-product of modern industrialization society. It occurs due to industry and the number of motor vehicles present in the cities. This problem is more prominent in large cities that have a warm, sunny and dry climate. Photochemical smog is also referred to as oxidizing smog and the reactions of oxidation has described in different ways. Therefore, it is seen that photochemical smog is a kind of air pollution.
FAQs
Q1. What are the main reasons for Photochemical smog?
Ans. The smog turns into Photochemical smog when comes to exposure to sunlight. It reacts with the pollutants in the air and the pollutants like hydrocarbon reacts with sunlight and formed thick clouds of smoke. It does not move much and dissipates slowly and the cloud can cover a town and it can result in no sunlight in the morning.
Q2. What are the important steps for reducing smog?
Ans. Some steps can help in reducing the effects of smog. The plantation of more trees can help in the purification of the air. The concern of the citizens about reducing the number of automobiles can help in reducing smog.
Q3. What are the main compositions of photochemical smog?
Ans. Nitrogen dioxide and nitric oxide are the two main compounds of nitrogen that helps in the creation of photochemical smog. They are produced from the burning of the fossil fuels. They are released due to events such as the eruption of volcanoes and the occurrence of forest fires.
Phthalimide
Introduction
The acid called Phthalimide is a compound that is very organic in nature. They are a part of a certain acid that makes for a part of phthalic anhydride. The formula of this acid is known as . The source of this product helps to cover a source of ammonia
What is Phthalimide?
Phthalimide is a product that comes from the part of organic chemistry. This compound is slightly acidic in nature as it is a derivative of phthalic anhydride. The derivative is imide in nature as they are going to fall under the functional group of the organic compounds. This type of organic compound is very sublime by nature. 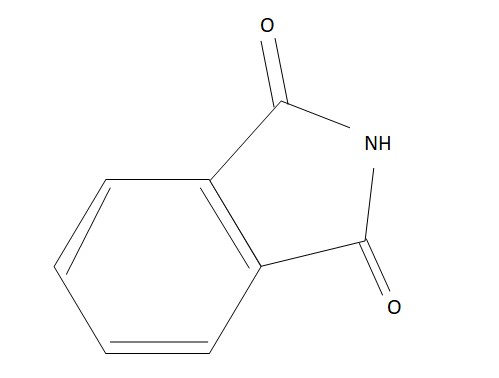 This is the reason why they are white this makes them very soluble in a solvent like water. This white compound is soluble in a bowl of water only if some kind of product that is used is very basic. This acid is formed as a base that has a decent trait of conjugation as they are going to have a very stable resonance. This acid comes from the group that has a proper site which is acidic. In this condition, these acids make a base of hydroxide that is very strong.
This is the reason why they are white this makes them very soluble in a solvent like water. This white compound is soluble in a bowl of water only if some kind of product that is used is very basic. This acid is formed as a base that has a decent trait of conjugation as they are going to have a very stable resonance. This acid comes from the group that has a proper site which is acidic. In this condition, these acids make a base of hydroxide that is very strong.
Figure 1: Phthalimide structure
Preparation of Phthalimide
The preparation of phthalimide is done by putting a certain amount of heat on a phthalic anhydride. There is a proper mix of aqueous ammonia along with this solution of phthalic anhydride. There is a different approach followed by many to produce phthalimide.
In this method, the anhydride is fused with a certain amount of ammonia when it is in its carbonate form. This is the alternative approach that is able to make phthalimide which is slightly acidic. The form of this acid is called ethanolic potassium hydroxide which looks for the formation of potassium Phthalimide.
Properties of Phthalimide
The properties of Phthalimide are listed below in the form of points. There is a vast range of properties that are present in phthalimide. They are listed down under this section –
The matter is very white and they are also solid in composition.
The mass of this organic compound is 147.33 grams per molecule.
The melting point of this organic compound is 238° C and the point where this acid starts to boil is 336° C.
All of these points are falling under the physical properties of this acid in its organic compound. The properties that are going to come after this one are associated with chemical aspects. This is the reason why all the points under this section are going to show the same.
This organic compound is very acidic and the pH value of this acid is 8.3.
The resonance of this organic compound is always stable by nature.
The derivative of this product shows it is very imide by nature. This means the acid falls under the functional group of organic chemistry.
The acid has a few heteroatoms that are present in it when the compound is slightly acidic.
When this chemical compound undergoes the process of reaction with a base it can make a grain of salt.
There are two groups of carbonyl present in this acid. These two groups of carbonyl are very electrophilic in nature. This is the reason why the concentration of this acid becomes very high.
These are the chemical properties of phthalimide that can be observed when the acid starts to react with some chemicals.
Uses of Phthalimide
Figure 2: Medical usage of Phthalimide
The uses of phthalamide are as follows -
They are used in the chemical industry where ammonia is produced.
The organic acid is highly used in industries where cloths are coloured by using the process of dying.
The acids are highly used in the production of peptides by carrying out a process of synthesising certain chemicals.
The acid is organic this is the reason why they have a huge role in the pharmaceutical industry.
Health Effects of Phthalimide
The health of this acid is that it causes some problems in breathing. The other problem is that it causes some irritation in the eyes. When a small drop of the acid falls on the skin they cause some minor burns. The effect of this acid on human health is minor as all it does is give some irritation to the organs mentioned above.
Conclusion
In this tutorial, all the learners are able to go through the phthalimide. The tutorial has a proper distinction of classes in order to help all the learners get a proper jest of the concept. One of them is here to deal with the physical traits that are found by the experts. The industrial uses of the chemicals and effects of this acid on the health of human beings are used to conclude the tutorial.
FAQs
Q1. What are the melting and boiling point of this organic compound?
Ans. The melting and boiling point of this organic compound are 238° C and 336° C. These are the readings that were observed when this organic acid was used observed.
Q2. How is the phthalimide acid used to make salt?
Ans. The process of making salt by the phthalimide acid is done by mixing base in a chemical form. The base has to be very strong by nature in order to make this happen.
Q3. What is the industrial use of phthalimide?
Ans. The commercial use of phthalimide is done in the industry where cloth are coloured with certain dyes. Another industry where this organic acid is used is in the chemical industry where ammonia is used.
Photochemical Reaction
Introduction
The Photochemical Reaction is quite different from a dark reaction, as, in a dark reaction, the thermal reaction concerns the ground state of electrons present within the molecule of a chemical compound. The Photochemical Reaction is responsible for playing a crucial role in defining the nature of chemical species. The chemical species include pollutant species present within the atmosphere. However, the Photochemical Reaction takes place due to the solar radiation within the atmosphere.
Defining Photochemical Reaction
Photochemical Reaction is nothing but a chemical reaction that takes place when triggered by light radiation from a light source. This results in the electrons within a molecule of a compound getting excited as a result of the absorption of heat energy.
However, in this reaction, detailed characterisation is considered as there occur significant changes in the primary events of Photochemical Reaction.

Figure 1: Multiple colourful close up photochemical reaction in glass vial under UV light in a dark laboratory
These stages show the pathways for the chemical reactions that are taking place. Photochemical Reaction, supports the sustenance of life on earth. The renowned scientist named Trommsdorff was the first one to have been able to describe photochemical reactions. He conducted this reaction through the experimentation of the two crystals of satonin when they are exposed to the light of the sun. this was proved as the crystals turned into a yellow colour followed by bursting out.
Types of Photochemical Reaction
Varied types of reactions are noticed in the aspect of photochemical reactions that includes, photo dissociation, which is expressed as
The next type of reaction is rearrangements of photo-induced or better known as isomerism, that is expressed as
Images Coming soon
The next reaction is known as photo addition that is expressed as
The other reaction is photo substitution that is
Lastly, the reaction of photo redox that is expressed as
Application of Photochemical Reaction
The reaction of Photochemical Reaction is quite essential as it is used for several industrial purposes. This reaction is majorly used in the making of Benzyl Chloride. For the production of the organic molecules that are synthetic in nature, this reaction is effective. In the preparation of varied drugs for anti-malarial, photochemical reaction is used.
Examples
Several examples are noticed for photochemical reactions, such as in the process of photosynthesis that takes place in plants when they make use of the sunlight for conversion of the carbon dioxide into products consisting of oxygen as well as glucose. Other examples include the ozone layer protecting the earth from ultraviolet radiation. The harmful UV rays that cause harm to DNA and skin cancer are due to the photochemical reactions that take place.
Laws Associated with Photochemistry
The laws that is associated with Photochemistry caters to Grotthuss-Draper law and Stark–Einstein law. In Grotthuss-Draper law, states that light needs to be absorbed by chemical substances.
However, the reaction is chemical in nature. Based on the Stark-Einstein Law, each light photon is to be absorbed by the stated chemical system. In this system, one or more gets activated by the reaction of photochemistry.
Occurrence of Photochemical Reaction in Photosynthesis
The process of photosynthesis refers to as the final property that is crucial to the sustenance of life on earth. This process is photochemical in nature that is conducted by green plants that involve green plants, for example, algae and seaweeds. In certain specific bacteria, the process is noticeable where the conversion of carbon dioxide to carbohydrates occurs as a result of the absorption of solar energy. Plants are known to make conversion of light energy into chemical energy. This energy gets stored in the plants in the form of carbohydrates by the effective usage of water as well as carbon dioxide, thereby releasing oxygen as a by-product.
More to this, the life of animals is sustained through the consumption of both oxygen and carbohydrates. However, both these products are prepared by the plants with the help of this reaction. This results in the active excitement of the electrons resulting in the transformation.
Conclusion
In this tutorial, the focus has been given on understanding the reaction of photochemical that occurs within nature. Most commonly the reactions occur when the electrons within the molecules get excited due to the absorption of heat energy in the form of light. In this reaction, sunlight is quite essential and in this reaction, molecules of the chemical compounds get segregated as well as modified thereby mixing with one another and resulting in the formation of new products.
FAQs
Q1. What is defined as Photochemical dissociation?
Ans. Photochemical dissociation also known as photolysis or photodecomposition is stated as a chemical reaction where a breakdown of photons occurs for a chemical compound. In this reaction, one to more photons tends to react by interacting with a target molecule. However, in the case of visible lights, there is no limitation to photo dissociation.
Q2. What initiates the reaction of photo dissociation?
Ans. The chemical reaction of photo dissociation tends to get initiated by the absorption of heat in the form of light rays. This results in the absorption of the molecules of light that is referred to as the development of the state of excitation. This state consists of varied chemical as well as physical properties that are quite different from the properties that were exhibited by the original molecules.
Q3. What is defined as the primary photochemical process?
Ans. The primary photochemical process is stated as photolysis; however, the intermediate consequence as a result of light absorption is the reaction to photochemical. However, the secondary reactions are known as chemical shifts that occur subsequently.
Phosphorus Trichloride
Introduction
Phosphorus trichloride is considered a fuming liquid and the chemical formula of this element is . A colourless liquid is like transparent liquidity in terms of motile. This chemical element is mainly manufactured with the help of burning molten white phosphorus that is kelp in the contact with a strong base of nitrous acid.
What is Phosphorus Trichloride?
Phosphorus trichloride is mainly available in a liquid state and it can be poisonous as well as volatile in terms of atmosphere. The reactive power of this chemical agent is very high and it displays its explosive nature during the time of reaction with the water. The IUPAC name of this chemical particle is Trichlorophosphane and it is mainly obtained through the process of synthesis of different organic substances. It is very toxic in nature and corrosive as well. Another identity of the chemical particle is phosphorus chloride.
Structure of Phosphorus Trichloride
The chemical equation of phosphorus trichloride is . In the structure of this chemical element is where hybrid orbitals of these properties overlapped with the orbitals of P of chlorine for forming sigma bonds of 3P-CL.
Figure 1: The structure of phosphorus trichloride
The fourth orbitals of the hybrid include a single pair of electrons. The structure of the pyramid is seen in the chemical bond of this element.
Phosphorus Trichloride: Physical properties
Some significant physical properties can be seen in the chemical compound of this element.
It is a colourless liquid and oily in nature. It is slightly yellow in colour.
The boiling point of this chemical agent is 347 K while the melting point is 161 K.
The molar mass of this chemical particle is 137.33g/mol and the density of this agent is 1.574 g/cm3.
The pungent odour of this chemical agent is very high and the reaction power of this element is greater than other components.
It is very similar to the acidic base of HCL and it is constantly fuming that is mostly seen in moist air.
The pressure of vapour of the chemical agent is 13.3kPa and the index of refractive is 1.5122.
The moment of the dipole of the chemical particle is 0.97D.
Phosphorus Trichloride: Chemical Properties
Phosphorus trichloride is highly reactive with water and after the reaction; it produced phosphorus acid. The reaction can be presented as
The combination of this particle with oxygen forms phosphorus oxychloride and this reaction can be presented as
The reaction of this particle with halogens as well as sulphur monochloride forms another element of the phosphorus group that is phosphorus pentachloride and the equality of this reaction is
Figure 2: Chemical properties of phosphorus trichloride
This chemical element has high reactive power and it is reacted with different chemical compounds like OH groups and the chlorine group. The reaction can be presented as
The readily oxidise of these chemical agents is to the phosphorus derivatives. It mainly undergoes the substitution reaction both in the organic as well as in terms of inorganic reactions.
Application of Phosphorus Trichloride
The application of this chemical compound can be seen in different industries for different purposes.
This chemical agent acts like a nucleophile and because of the existence of a single pair; this can connect the pair with the electron deficient compound.
Phosphorus trichloride is also acted like an electrophile and because of the existence of zero orbital of d; this particle easily accepts the electrons from the rich compounds. It can expand the valency of own up to 5.
The chemical reaction comprises commonly undergoes with the reaction of redox.
The toxic nature of this chemical agent is very high and it is able to react violently with water as it includes the atom of oxygen as well as hydrogen.
At the time of chemical reaction, this element produces a great amount of heat that increases the temperature of adjacent particles.
Irritation to the eyes can be seen during the application of this chemical agent. It can affect the skin as well as the respiratory system of the human body along with other animals.
Conclusion
In the production of phosphorus oxychloride with the help of oxidization in the presence of oxygen, phosphorus trichloride can be applied. In the current situation, this molecule is severally applied as chlorinating particles in the conversion process of different types of organic acids. In the production process of phosphorous pentachloride, phosphoryl chloride as well as thiophosphoryl chloride it is mostly used. This agent produces a systemic effect with the absorption with the help of the skin within the bloodstream.
FAQs
Q1. What is the formula of phosphorus trichloride?
Ans. The formula of this agent in terms of chemical properties is and after the hybridization of the phosphorus, it becomes . The chemical structure of these particles is trigonal bipyramidal as it undergoes the hybridization of . The bond angles of the structure of this chemical agent are lower than 109°.
Q2. What is the preparation process of phosphorus trichloride?
Ans. The preparation of this chemical agent can be presented as . It is able to gain this formula with the help of a reaction with thionyl chloride that starts in the presence of white phosphorus and this equation is presented as .
Q3. What is the complexity and solubility of this agent?
Ans. The complexity of this chemical agent is 8, that are generally higher than the other molecules in this group. This chemical molecule has great soluble power and it is easily soluble in water but not in the acid-base.
Phosphorus Pentachloride
Introduction
Phosphorus Pentachloride can be generated through an effective and common procedure of dry chlorine gas action on phosphorus trichloride. In the solid-state Phosphorus Pentachloride, remain as . The boiling point of Phosphorus Pentachloride is nearly 166.8°C and the melting point of Phosphorus Pentachloride is about 160.5°C.
Phosphorus Pentachloride
Phosphorus Pentachloride has a crystalline salt-like structure that gets dissociates partially in solution mainly in polar solvents like nitrobenzene. Phosphorus Pentachloride is determined as the solid having a pale greenish-yellow colour, which can be specified with the formula PCl5. The preparation methods of the Phosphorus pentachloride depict that the compound can be easily prepared through dry chlorine action in phosphorus trichloride.
The law of mass action highlights that Phosphorus Pentachloride gets vaporized without the action of dissociation in the air that involves the gas of chlorine or phosphorus trichloride. In the presence of these products, the equilibrium of the dissociated slightly shifts to its left.
Structure of Phosphorus Pentachloride
In order to identify the solid compounds, it is very important to understand their structure and properties. The shape of Phosphorus Pentachloride is trigonal bipyramid  and it has a greenish-yellow crystalline solid appearance (Xu, Wang & Wang, 2019). The hybridization that can be seen in the compound of phosphorus pentachloride is sp3d. The compound has 2 axial bonds and 3 equatorial P−Cl bonds.
and it has a greenish-yellow crystalline solid appearance (Xu, Wang & Wang, 2019). The hybridization that can be seen in the compound of phosphorus pentachloride is sp3d. The compound has 2 axial bonds and 3 equatorial P−Cl bonds.
Figure 1: Structure of Phosphorus Pentachloride
Various methods are involved in the preparation of this organic substance as stated below.
Properties of Phosphorus Pentachloride
Phosphorus Pentachloride is referred to as a reactive chemical agent that can causes weakness, nausea, headache, dizziness, and vomiting when the humans get exposed directly to the compound.
Phosphorus Pentachloride gets easily vaporized without any dissociation with the air of chlorine gas or phosphorus trichloride. The equilibrium of dissociation gets shifted gradually to its left side due to the presence of a substance in the reaction.
The physical properties of Phosphorus Pentachloride are stated in the table below.
| Properties of Phosphorus Pentachloride | |
|---|---|
Formula of Phosphorus Pentachloride | |
Density | |
Molecular weight | 208.24 g/mol |
Odor | Irritating and pungent smell |
Shape of Phosphorus Pentachloride | Trigonal bipyramid |
Boiling point | 166.8°C |
Melting point | 160.5°C |
The chemical properties of Phosphorus Pentachloride can be understood through some reactions as mentioned below.
Dissociation of Phosphorus pentachloride can be written as:
Reaction with water
Reaction with metal:
Reactions with phosphorus pentoxide:
Reactions with sulfur dioxide:
Uses of Phosphorus Pentachloride
Phosphorus Pentachloride has several common uses as it acts as an effective reaction in medical and industrial fields. The compound is highly used as the chlorinating agent and helps in disinfectants of floors, furniture, and equipment in medical sectors.
Figure 2: Uses of Phosphorus Pentachloride
In order to manufacture the two major components, include penicillin and cephalosporin in several pharmaceutical industries. Phosphorus Pentachloride also help in the production of acid chlorides. The compound is generally utilized as an effective catalyst in the production of acetyl cellulose. Phosphorus Pentachloride also help Phosphorus Pentachloride is also used in the case of condensation reactions and cyclization.
Effects of Phosphorus Pentachloride
Some common effects of Phosphorus Pentachloride on living beings and nature are stated below.
Phosphorus Pentachloride is referred to as a reactive chemical agent that can causes weakness, nausea, headache, dizziness, and vomiting when the individual gets exposed directly to it.
Excessive inhalation of Phosphorus Pentachloride can cause damage to the kidneys, liver, cardiovascular system, and respiratory tracts of humans.
Irritation in the nasal cavity and throat can occur if the individual works and remain exposed to Phosphorus Pentachloride for a long period of time.
A person can experience bring sensation, and irritation in the eyes that causes severe eye damage.
Shortness of breath and damage to the lungs occurs when an excess amount of Phosphorus Pentachloride is breathed by a person. This case leads to excess fluid accumulation in the lungs that gradually affects other respiratory organs and causes severe disease determined as pulmonary oedema.
Development of phlegm and cough, in bronchitis, also occurs due to excessive consumption of Phosphorus Pentachloride.
Conclusion
Phosphorus Pentachloride has the shape of the trigonal bipyramidal geometry in cases of gaseous and liquid states. Excessive consumption of Phosphorus Pentachloride can cause damage to the kidneys, liver, cardiovascular system, and respiratory tracts of humans. The compound is widely used in various medical sectors as sanitizing agent and disinfectant.
FAQs
Q1. What are the major physical properties of Phosphorus Pentachloride?
Ans. Phosphorus Pentachloride include several physical and chemical properties that are often determined to be harmful material for human health. The formula of Phosphorus Pentachloride can be written as . The boiling point of Phosphorus Pentachloride is nearly 166.8°C and the melting point of Phosphorus Pentachloride is about 160.5°C.
Q2. What is the significance of Phosphorus Pentachloride?
Ans. The major significance of Phosphorus Pentachloride include it acts as an effective catalyst in the production of acetyl cellulose. Phosphorus Pentachloride also help Phosphorus Pentachloride is also used in the case of condensation reactions and cyclization.
Q3. What are the risks that are caused by Phosphorus Pentachloride to the health of humans?
Ans. Shortness of breathing occurs when an excess amount of Phosphorus Pentachloride is consumed by a person. The excess inhalation of the compound can affect the tissue and bloodstream that gradually affects the lungs causing heart disorders. Excessive accumulation of fluid takes place in the lungs that gradually affects other respiratory organs and causes severe disease determined as pulmonary disorders.
Measurement of Enthalpy and Internal Energy Change
Introduction
Normally, the measurement of enthalpy and internal energy change is carried out by an experimental approach. This approach is known as calorimetry which is an important part of chemistry. It is mainly a technique which is developed on thermometric procedures. Here, a calorimeter is the vessel in which the procedure happens. In this part, the vessel is submerged in a known liquid volume.
What is Enthalpy?
Enthalpy is the heat energy and it is absorbed during a chemical reaction progression. In this part, enthalpy is represented by H and it indicates the energy amount. The change of enthalpy is described through ΔH where delta refers to the change. It consists of a unit which is joules or kilojoules.
It can simply be described that the sum of internal energies of the system is enthalpy. The cause of this factor is that a change in internal energy takes place at the time of chemical reaction.
Here, the formula is
In this part, H is enthalpy, U is the sum of internal energy, P is the pressure of the system, and V is the volume of the system. Therefore, it is said that enthalpy is the addition of internal energy.
What Is Internal Energy?
Internal energy is considered the addition of potential and kinetic energy of that particular system. In this part, the stored energy is potential energy which is released due to movement of molecules. It is the kinetic energy of the particle and internal energy is represented by U. The change in internal energy is described through the symbol ΔU.
The factors regarding this matter say that at a constant pressure internal energy and enthalpy are the same for a particular system. The change in this part can happen in two ways (Zhang, Shu & He, 2019). One happens because of the transfer of heat and the second happens because of doing work. The system can absorb or release heat and the change happened.
It is presented through the following equation:
Here, ΔU is the internal energy change, q is the transfer of heat, and w is work done by or on a system.
Enthalpy Change Measurement
The measurement of Enthalpy Change is described by expressing ΔH and it is the flow of heat at constant pressure. It is calculated by the use of a calorimeter of constant pressure. The factor shows the direct value of delta h enthalpy. The commercial calorimeters here also functioned on the similar principle.
They use the factor in a different way for describing the measurement of enthalpy change. The matter describes that a certain amount of energy is required for breaking the bond between one-mole molecules. The atomization enthalpy is needed for the conversion of any material intro gaseous atoms. Differently, it is said that sublimation enthalpy is the process where heat is essential for converting a mole of a substance from a solid to a gaseous state.
Measurement of Change in Internal Energy
Image: Bomb calorimeter vector illustration - Constant volume type to measure heat internal energy
Internal energy change is referred to the change of energy at constant volume. For example, in this part, a bomb calorimeter is applied to measure internal energy change. This process used a steel vessel and it was seen as emerging in a water bath. It is happened considering the fact that no heat is lost to the surrounding.
It is observed that a combustible substance is mainly burnt in oxygen gas. The oxygen gas is supplied in the bomb here. It is seen that energy changes associated with the reaction. It can be measured in a constant volume because it does not change completely. The volume here remains constant and that is the reason the work is done at a zero system.
Strategies
The strategies show different factors like
It describes the calculation of a solution’s mass and its volume and density.
The matter of evaluation of change in temperature of the solution is also described here.
It determines heat’s flow.
It uses KOH’s molar mass.
Uses of Bomb Calorimeter
The Bomb Calorimeter is an important part of thermodynamics. It is seen that this type of calorimeter is utilised to decide heat release amount in burning reaction. It is also important for finding the calorific food value. Bomb calorimeters is most important for the industries and it is seen that it is mostly used in food processing, metabolic study, and testing of explosives.
It is very important for determining the heat which is emitted from a given quantity of biomass sample combustion. It is also used for calculating the HHV of that biomass fuel. It is considered to be an instrument for measuring heat. The instrument is very useful in calculating the calorific value of solids.
Conclusion
The research shows that change in internal energy is mainly the sum of heat transmission and work done. The most important factor here is that internal energy and heat flow is related to the system’s enthalpy at constant pressure. Here, the heat is evaluated by using known heat capacities of the calorimeter and liquid. This mainly talks about experimental technique which is known as calorimetry.
(FAQs)
Q1. What does enthalpy refer to?
Ans. Enthalpy is the released heat and it is absorbed during chemical reactions. In this part, H is the required energy and it presents the change in enthalpy. Here, delta represents the change and joules or kilojoules are used as units.
Q2. How does the measurement in the calorimetry process done?
Ans. Here, measurement is done with the use of two conditions and it is essential for this process. The conditions are at constant volume and at constant pressure. These are the most important part of the calorimetry process.
Mass Spectrometry
Introduction
The mass spectrometers are mainly utilized in order to detect several unknown components through molecular weight and it also helps in the determination of phenomenon. This process also helps to quantify the already detected components and determine the structure and chemical properties of the molecules that are present in the given sample. The mass spectrometer generally performs through three major steps including Ionization Source, Ion Detection System, and Mass Analyzer. The two instruments that are utilized in various technical fields to study mass spectrometry is also determined as mass spectroscopy, are mass spectrometers and mass spectrographs.
What is Mass Spectrometry?
Mass spectrometry, also denoted as mass spectroscopy. This mechanism mainly utilizes an analytic technique through that the chemical substances are determined with the help of the sorting of gaseous ions in the electric and magnetic fields depending on their mass-to-charge ratios. The gadgets that are often utilized in the study of mass spectrometry include mass spectrometers and mass spectrographs. The instruments are being operated based on the principles that help the ions to move. The ions may get deflected through the electric and magnetic fields.
Working Principles of Mass Spectrometry
Mass spectrometry uses some sources like Gas phase methods, Desorption methods, and Spray methods. Every mass spectrometer contains 3 major components including Ionization Source, system of Ion Detection, and Mass Analyzer.

Figure 1: Working principle of spectrometry
Ionization Source: The particles that are moved to gas-phase ions can move and be manipulated through external electric and magnetic fields. The techniques that are used in the lab are determined as nanoelectrospray ionization. This technique is the same as the process of how cars are painted in their industries. This method of ionization is basically utilized in order to create positive or negative charged ions. This phenomenon highly depends on the experimental requirements. The Nanoelectrospray ionization, helps in coupling the outlet column of the small-scale chromatography, which directly inserts into the inlet of a mass spectrometer. The flow from this column is then passed through a needle which is of 10-15 um at its tip.
Mass Analyzer: After the stage, ionization source, the ions get sorted and separated on the basis of mass-to-charge (m/z) ratios. Various numbers of mass analyzers are presently required in this process, each of which trade off on the basis of speed of operation, resolution of separation, and many other operational requirements. The specific kind of mass analyzers are often utilized in several technological fields. The mass analyzer often appears in relation to the ion detection system.
Ion detection system: The ions that get segmented and measured and sent to a data system. In this data system, the m/z ratios remain stored which remain associated with the relative abundance. The mass spectrum is generally denoted as the m/z ratios of the ions that are present in a sample plotted against their intensities. Each peak in a mass spectrum will then show a component of unique m/z in the sample. The heights of peaks will then connote the relative abundance of several components present in the given sample.
Types of Mass Spectrometry
The word mass spectroscope is utilized in order to involve both kinds of devices. In this context, electrical detectors are presently used in several engineering or technical fields. The detectors are often used in the field which is usually determined as mass spectrometry (robynsrevison, 2022). There are several types of mass analyzers used widely including Time-of-flight (ToF), Magnetic sector, Ion trap, Quadrupole, and Tandem mass spectrometry (tandem MS), and Orbitrap. The effective methods that are often used in Mass Spectrometry are Gas phase methods, Desorption methods, and Spray methods.
Stages of Mass Spectrometry
The mechanism of Mass spectrometry, is often required in order to measure the mass of several molecules within a provided sample. The 4 major stages of mass spectrometry include ionization, acceleration, deflection, and detection.
The ionization stage, uses the sample that is vaporized before it is passed within an ionization chamber where it is then bombarded by a stream of electrons that is emitted through an electrically heated metal coil. The positively charged ionization chamber usually repels the positively charged ions, and it accelerates to 3 negatively charged slits with progressively reducing voltage. Deflection, is the stage where the stream of positively charged ions is deflected through the magnetic field. The final stage is denoted as detection, where the beam of ions pass-through the mass analyzer and gets detected through a detector based on the mass-to-charge ratio (m/z).
Conclusion
The chemical analysis which is used in the measurement of the mass-to-charge ratio (m/z) of atoms and molecules in a sample is determined as the Mass spectrometry. The 2 main instruments that are often used in this mechanism differ only in the way in which the sorted charged particles are often outlined. The two instruments are mass spectrometers and mass spectrographs. In the mass spectrometer, they are often detected electrically, and in the mass spectrograph through photographic or other nonelectrical means.
(FAQs)
Q1. What happens in the deflection stage?
Ans. The deflection stage is the final stage. In this stage, the ion hits the detector, and the charge is neutralized through an electron that is jumping from the metal onto the ion.
Q2. What are the major stages of Mass Spectrometry?
Ans. The mechanisms of mass spectrometers are widely used in various fields of technology as they play a significant role in this world of technological advancement. The 4 major stages of mass spectrometry include ionization, acceleration, deflection, and detection.
Q3. What are the instruments that are used in the study of Mass Spectrometry?
Ans. The study of mass spectrometry is also determined as mass spectroscopy. The two instruments that are utilized in the study of Mass Spectrometry are mass spectrometers and mass spectrographs.
Metal Band Theory
Introduction
The molecular orbitals that help in the formation of the band seem to have the same energy as the atomic orbitals. This theory mainly describes the fact that electrons can jump from the valence band to the conduction band. This process happens when the temperature remains ordinary because it can help the solids to conduct electricity. The conductivity mainly depends upon the gap between the valence band and conduction band.
Description of Metal Band Theory
Band theory is considered a concept that focuses on the matter of solids. This theory is also known as zone theory and it shows the relationship between valence and conduction bands. In this part, the valence band describes the orbitals of the valence shell and they contain electrons.
On the other hand, the conduction band is formed of orbitals. Therefore, it can be said that the orbitals of the conduction band are empty. The development of this theory occurs by gathering the knowledge that is witnessed during the quantum revolution in science. As per the view of this theory, atomic orbitals are an important part of the electron particles. It helps to give a shape to the distinct arrangement of the energy levels.
Images Coming soon
Figure 1: Energy Bands for Solids
The theory shows that solids electrons jump from valence band to conduction band. This happens when the temperature stays ordinary and in this state solid conducts electricity. This matter of conductivity mainly depends on the gap between the valence band and conduction band. The most important factor here is that when the gap between these bands is equal to or more than 5ev then the material will behave as an insulator.
It also exposes the fact that when the energy different lies equal to or less than 3ev, then the material is named as semiconductor. In contrast, the overlapping of the valance and conduction band is called conductors. The reason behind this factor is that electrons can jump from valance to conduction band and thus conduct electricity. The semiconductors are the factor where few electrons can jump from valance band to conduction band.
Valence Band, Conduction Band, and Forbidden Gap
The Band Theory of Metals is dependent upon the valance band and conduction band. This theory is also known as the band theory of solids or zone theory of solids. The matter defines the aspects of conductor, semiconductor, and insulator very clearly. The description of the terms is given below-
Valence Band: This band is built up of the specific valence shell orbitals and it consists of electrons in them. An example of this factor is that a sodium valence band is made up of 3s1 orbital. It consists of the configuration of the electron of sodium and it is 1s2, 2s2, 2p6, 3s1.
Conduction Band: the band of conduction is built up of orbitals which are unoccupied by electrons. They are seen either in the valence shell or in the higher unoccupied shell. That is the reason to develop the conduction band empty. This factor describes that the highest energy band is known as valance band.
Forbidden Gap: Forbidden Gap is an important part of the band structure of sodium. In this part, this gap is considered the energy difference between the valance band and conduction band.
The matter regarding this theory also describes some other points such as conductors, semiconductors, and insulators. Conductors are the materials which allow electricity to pass through them. Some examples of this factor are zinc, iron, copper and many more.
Semiconductors are the materials which show the conductivity between conductors and insulators. Some factors can be given as examples and they are As, Ge, and Si. Insulator is defined as the materials that hinder electricity to pass through them.
Examples of the insulators are stones, grass, wood and many more.
Examples of Band Theory
The explanation of band theory can be defined with the example of sodium and it will help to understand the matter very easily. The atomic configuration of sodium is1s2, 2s2, 2p6, 3s1. It consists of unpaired electron in the 3s orbital and they overlap with each other.
The process has happened for the formation of molecular orbitals. The energy difference presents the energy spread between the bonding orbital and anti-bonding orbital. The highest gap between the valence band and the conduction band hinders electrons to jump from one band to another. This incident is noted as less or no conductivity.
Conclusion
The tutorial describes that metal band theory is based on the valence band and conduction band. They are the main aspects of this theory and that is the reason this theory is also called the theory of solids or zone theory. The theory gives the description of different aspects which are important for this factor. The aspects are conductors, insulators, and semiconductors. Other important factors of this theory include the forbidden gap, valance band, and conduction band.
(FAQs)
Q1. Who gives the proposal of the metallic band theory?
Ans. The revolution of quantum helps in the development of metal band theory. It is seen that in the year 1928, Felix Bloch applies the idea of quantum theory. Later, in 1927, Walter Heitler and Fritz London discovers the idea of this theory.
Q2. What is the reason for the formation of the band gap?
Ans. The gap is formed when two bands are not wide enough to span the full range of electron energy levels. The band gap is the gap between the valance band and conduction band.
Q3. What is the benefit of band theory?
Ans.Band theory helps to know the difference between conductor, semiconductor, and insulator. The difference is made by plotting available energies for an electron in a material.
Polystyrene
Introduction
The manufacturing process of different types of lightweight and flexible items is improved from before after considering the application of polystyrene. Styrene is the most effective monomer of polystyrene. Another term associated with polystyrene is ethyl benzene. Phenyl ethane and vinyl benzene are two other components in which all integrated precursors are included.
Definition: Polystyrene
Polystyrene is an important stiff, transparent and hard resin and is produced by the polymerization of styrene. At room temperature, a thermostatic polymer is found in a solid form. At the time of heating the component at a high temperature, polystyrene started to transfer into a liquid form. At a cooling time, this component becomes rigid.

Figure 1: Polystyrene PS plastic, Chemical structure
Polystyrene is a component that is insoluble in water. These are some other copolymers as well that are responsible for maintaining the componential attributes of polystyrene. The manufacturing process of some performance-efficient, cost-efficient and energy-efficient products is also associated with the functions of polystyrene.
Properties of Polystyrene
Polystyrene possesses some significant properties based on which its significance in preparing flexible and transparent items can be understood.
Polystyrene’s nature is non-polar.
The existence of polystyrene is found in an amorphous state. This component’s presence in some phenyl groups is the main reason behind this.
The melting point of polystyrene is 240°C.
The range of polystyrene dentistry is 1.05g/cm3.
The gravity range of polystyrene is 1.054.
Thermal conductivity is an important property of polystyrene whose value is 0.003W/m.K.
The optical property of polystyrene is also an important part as it is a very transparent polymer. The range of transmission is very high in relation to almost all wavelengths. The high brilliance of polystyrene is generated by its high refractive index.
Structure of Polystyrene
Polystyrene is known to be an important vinyl polymer in which a long hydrocarbon chain is found. Each carbon atom of polystyrene is integrally attached to the structure of polystyrene. The free vinyl process of polymerization is important as this monomer styrene is integrally attached.
The main components of polystyrene’s structure are carbon and hydrogen. Some of the atoms are attached to the carbon molecules. Some atoms are found as a bond between carbon and hydrogen. These structural components are helpful in presenting pure polystyrene, which is an important linear polymer. A very small amount of divinyl benzene is found in the structural component of polystyrene. The branches are induced, as not much divinyl benzene is capable of handling the polystyrene gel.
Usage of PolystyreneIn current industrial purposes and in some other areas as well, polystyrene is widely used.

Figure 3: Thermal insulation coatings with insulating panels in polystyrene and graphite for building energy efficiency
Some of the key usages of polystyrene are as follows:
In medical purposes, polystyrene is used significantly.
The sterilization procedure of test tubes depends on the usage of polystyrene.
Some diagnostic components are also made by using polystyrene.
Some parts of a car are also made of polystyrene. Such parts can include instrument panels, knobs and sound dampening foam.
The egg cartons and DVD cases are packed using polystyrene. Using polystyrene is safe in packing these as it can prevent damage and is less expensive.
The usage of polystyrene can provide chances to increase thermal insulation and therefore in freezers and refrigerators, polystyrene is an important component.
Some of the most required IT equipment is prepared by using polystyrene.
Parts of television and computers are also prepared by using polystyrene.
Polystyrene Safety and Food Packaging
Polystyrene possesses a very low tendency in relation to absorbing moisture. This is the reason, in packaging food; using polystyrene is beneficial and helpful. In the presence of benzoyl peroxide, polystyrene is prepared in which free radical addition is also integrated. As an important catalyst, benzoyl peroxide is an important component of polystyrene. The safety measure of polystyrene is used in packaging food.
According to the European Commission, the characteristics and componential attributes of polystyrene are fully safe when it comes in direct contact with food.
All types of food-borne illnesses can be easily reduced by considering a package, made of polystyrene.
All the packaging, made of polystyrene, is less expensive than any other materials.
Polystyrene can be easily reused and recycled and this is an important consideration for the continuous increase in polystyrene demand.
Conclusion
Polystyrene is a significant amorphous polymer, which is non-polar in nature. A very low temperature is required for softening polystyrene. At the temperature of boiling water, this component cannot stand. Polystyrene possesses no metallic sound when it is dropped. These characteristics state that in the current time, the usage of polystyrene for industrial purposes is very important. Air, being one of the main parts of polystyrene's composition, leads this to be very lightweight. Both the extruded and expanded polystyrene possess equal importance. The environment-friendly nature of this component is an important reason behind its increasing demand.
(FAQs)
Q1. Why is polystyrene popular in the food service industry?
Ans. In the food service industry, polystyrene is very much important as it helps in maintaining the safety of food items. In commercial alternatives as well, polystyrene is a useful component. The food service industry can be ensured regarding convenience and proper dining satisfaction.
Q2. What is the reason behind brittle polystyrene in nature?
Ans. The impact of chain stiffening on all benzene rings is an important reason behind the brittleness of polystyrene. This characteristic leads this component to produce the least sound when it is dropped.
Q3. What is extruded polystyrene?
Ans. A specific form of plastic is named extruded polystyrene, which is used in various forms. In almost all building materials and in the preparation of some storage containers extruded polystyrene is very important. All the extruded polystyrene possesses a significant form which is like a plastic-like material.
Materialsduction
Materials accumulate for the composition of all the objects in the surroundings. The associated properties of the materials are different from one another. Most of the finished goods are made of up raw materials that have their unique traits which are mixed to make a final unit of product. The classification of the properties is done based on various physical as well as chemical properties.
What is a Material?
Materials are referred to as the building blocks of the finished products available in the market. The material in itself is a broad spectrum to discuss. The classification of the materials is conducted based on a certain set of properties. All the materials display a certain set of physical properties that include hardness, stiffness, strength, thermal conductivity, heat capacity, permeability, and magnetism.
The science associated with the study of materials is termed material science. The materials available in the open have a diverse range of uses. The classification is based on their use in their industries. The process of making materials to be utilized in the course of certain applications is called material selection.
Properties of Materials
Materials have a diverse range of properties. However, for scientific purposes the physical and the chemical properties of a matter are essential to be considered. The physical properties of material are subcategorized into electric, magnetic, thermal and mechanical properties.
The chemical properties of a particular material include their distinct states, that is, solid, liquid, gaseous, solubility, pH, reactivity, the tension of surface, energy of the surface, corrosion, etc. There are distinct sets of properties based on the mechanical aspect, elasticity, plasticity, ductility, durability, brittleness, hardness, malleability, resilience, stiffness, and viscosity.
Classification of Materials - Based on Physical Properties
Materials can be classified based on a certain set of physical properties.
The properties are mentioned below:
Appearance: Materials can be differentiated from one another based on the looks of a material Some of the materials are known to reflect light and shine and they are called lustrous.
Images Coming soon
Figure 1: Material classification based on appearance
On the contrary, some objects do shine graphite and wood are common examples of this kind of material. Iron, gold, diamond, and cooper are lustrous and they are called metals.
Hardness: Materials that can be compressed or can be scratched easily are considered soft materials. On the other hand, the materials that are hard to compress of scratch are referred to as hard substances.
Images Coming soon
Figure 2: Material classification based on appearance
Transparency: Materials that cannot be seen past are referred to as opaque objects. For example, trees, iron sheets, etc. On the other hand, certain objects can see through easily, these substances are called transparent objects. For example, glass. Materials that allow partial visibility through them are called translucent materials. For example, oily papers.
Classification of Materials - Based on Atomic Structure
Based on the atomic structure of the materials they can be classified into several sections. These materials are further classified into metals, ceramics, and polymers.
Images Coming soon
Figure 3: Classification of materials
Metals: This section of materials classified the metallic elements like gold, iron, copper, nickel, and aluminium. These materials are hard, strong, and at times malleable. Certain meals like gold and silver are ductile and yet fracture and resistant.
Ceramics: This section of material has metallic and non-metallic substances. It mostly requires compounds like oxides, nitrides, and carbides. These products in general are strong and also brittle and they can also break down fast.
Polymers: These substances include plastic and rubber products. Most common examples are polyethylene, polyvinyl chloride, and nylon. These materials are not particularly stiff or strong when compared to metals or ceramics. These products are known to have low density and are quite ductile. That is, these products can easily be moulded into variant shapes.
Definition of Raw Materials
The materials that are not thoroughly processed and are utilized to make a final product is referred to as raw material. It is the most basic form of material that is available in nature. These materials are mostly used for industrial purposes to make a final product.
Conclusion
Materials that are found in nature are the basis of the production of final products. These materials are further subdivided into various categories that assist in the making of final products. These products have their own set of distinct chemical properties where the materials are categorized based on their atomic structure and other essential properties. These are also categorized based on their particular physical properties. The materials that act as building blocks of the final products are classified as raw materials.
(FAQs)
Q1. What is the essential properties of materials?
Ans. Most of the substances are classified based on four distinct properties, that is, elasticity, where the materials elongate and return to their original shape once the force is removed. Thermal conductivity denotes the extent of heat that courses through a material. Density is the mass of material per cubic centimetre. Finally, ductility, is the property of the material to alter its shape without deformity.
Q2. What is the categories of raw materials?
Ans. Raw materials are subdivided into two categories direct and indirect materials. The direct materials are used in final products. Indirect materials are used in the process of making the final product. For example, crude oil, iron ore, water, mineral, etc.
Q3. What are composites?
Ans. Composites are formed when polymers and ceramics are mixed to form an entirely new product. The final product is called a composite. Most of these products are man-made, and a handful of the composites occur in nature. Fibreglass is an example of a composite. This product is strong, stiff and ductile.
Q4. What are semiconductors?
Ans. These are the substances that display properties intermediate between conducts and insulators. Some of the most prominent semiconducting crystals are Silicon and Germanium.

No comments:
Post a Comment