Organic compounds Classification, Functional group and Homologous series
Contents [hide]
Classification of organic compounds ( Hydrocarbons)
Organic compounds are defined as the hydrocarbons (compounds containing carbon and hydrogen) and their derivatives in which covalently bonded carbon is an essential constituent.
Organic compounds are classified as :

1. Open chain organic compounds :
Organic compounds in which the terminal C-atoms are not joined together are called open chain compounds. Eg.

The open chain organic compounds can be further classified as,
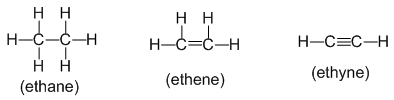
Alkanes : Alkanes are the saturated hydrocarbons with general formula CnH2n+2. They contain only carbon-carbon and carbon-hydrogen single bonds in their molecules. For example:
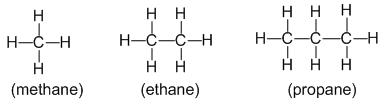
- Alkanes are also called paraffins (because they have a little affinity towards a general reagent. In other words, alkanes are less reactive substances. They undergo reactions under drastic conditions)
Note : Alkanes are called saturated because all the possible sites (i.e. 4) are bonded with other atoms.
But in alkenes and alkynes there is a possibility of addition of atoms or groups so they are called unsaturated.
Alkenes : Alkenes are unsaturated hydrocarbons with general formula CnH2n. They contain at least one carbon to carbon double bond in their molecules. For example:

- Alkenes are also called olefins ( i.e. oil forming because they form oily liquids on reaction with chlorine gas.
Alkynes : Alkynes are unsaturated hydrocarbons with general formula CnH2n-2. They contain at least one carbon to carbon triple bond in their molecules. For example:

2. Closed chain (cyclic) organic compounds :
Organic compounds in which the terminal carbons are joined together to form a cyclic structure are called closed chain or cyclic organic compounds. For exampne:
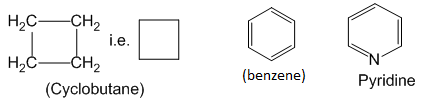
Cyclic organic compounds are further classified as – homocyclic and heterocyclic organic compounds.
Homocyclic compounds : Cyclic organic compounds in which the ring forming atoms are only carbon are called homocyclic or more specifically carbocyclic compounds.
Homocyclic compounds can be further classified as – Alicyclic and Aromatic compounds.
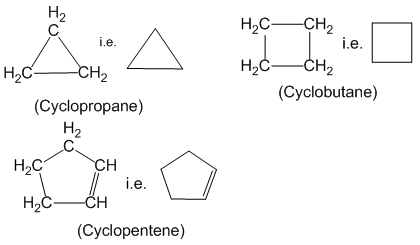
Alicyclic compounds :
Closed chain organic compounds whose properties are similar to open chain aliphatic compounds are called alicyclic compounds. For example:
Aromatic compounds :
Benzene and those cyclic compounds that chemically behave as benzene are called aromatic compounds. For example:

Aromatic compounds obey Huckel’s rule.
Note : Huckel’s rule :
Huckel’s rule states that a cyclic and planar molecule is aromatic if it contains 4n+2 delocalized π electrons, where n = 0,1,2,3,4,etc.
Examples : Benzene
Benzene is cyclic and planar and has cyclic overlap of p-orbitals. There are 3 double bonds i.e. 6 delocalized π – electrons, which is consistant with Huckel’s rule.
i.e. 4n+2 = 6
4n= 4
n = 1(which is an integer)
Therefore, benzene is an aromatic compound. It will show aromaticity.
Heterocyclic compounds :
Cyclic organic compounds in which at least one heteroatom (i.e. atom other than carbon eg. N, O or S ) is present as one of the ring forming atoms are called heterocyclic compounds. Examples :

Formula of organic compounds
• Molecular formula :
It represents actual number of atoms of all the elements present in one molecule of the compound. For example:
methane = CH4
ethane = C2H6
ethene = C2H4
benzene = C6H6 , etc.
• Empirical formula :
It represents simple whole number ratio of atoms of all the elements in one molecule of the compound. For example:
ethane = CH3
ethene = CH2
benzene = CH , etc.
• Electron-dot formula : In this formula valence electrons are represented by dots placed around the chemical symbol. It is also called Lewis formula. For example:
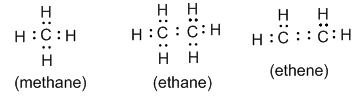
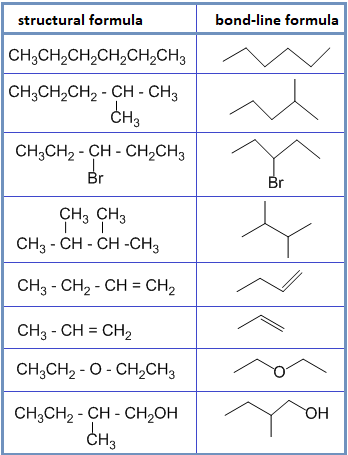
• Structural formula : It indicates how the atoms are bonded in a molecule of the compound. For example:
• Contracted or condensed formula :
It is the structural formula in contracted form to save space and time.


• Bond – line formula :
In this type of formula, carbon and hydrogen atoms are not shown and only hetero atoms are shown. The point of intersection represents carbon along with required number of hydrogen to satisfy the valency of carbon. For example:
• Spatial formula :
This formula represents the three dimensional shape or arrangement of atoms in the molecule. For example :

Functional group
An atom or group of atoms in a molecule which largely determines the chemical properties of the organic compounds is known as functional group. All the compounds having same functional group show similar properties and constitute a class or a family.
For example: organic compounds having – OH as functional group constitutes a class of compounds called alcohol.

Some other examples of functional group are :

Homologous series
The series of organic compounds having same general formula and similar chemical properties but different physical properties in which one member differs from other member by single – CH2 unit is known as homologous series. For example:
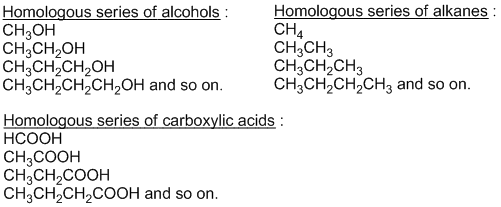
Each member of homologous series is called homologue and phenomenon of making homologous series is called homology.
Characteristics of homologous series :
- All the members of homologous series have same functional group.
- All the members of homologpous series have same chemical properties.
- All the members of homologous series can be prepared by a common method of preparation. Eg.

- All the members of homologous series can be represented by same general formula. Eg.
CnH2n+2 = Alkane
CnH2n = Alkene
CnH2n-2 = Alkyne
CnH2n+1OH = Alcohol, etc.
- Their molecular masses increases gradually hence their physical properties (eg. melting point and boiling point) changes gradually.
- Each member differs from the adjacent member by methylene (-CH2-) unit.
Q) Write down the 1st, IInd, IIIrd and IVth homologue of aldehyde homologous series.
Ist homologue = HCHO
IInd homologue = CH3CHO
IIIrd homologue = CH3CH2CHO
IVth homologue = CH3CH2CH2CHO
See the IUPAC Nomenclature of Organic compounds ……
Objective questions
1. Cyclic organic compounds possessing the properties of aliphatic compounds are called ___ compounds.
a. Aromatic c. Carbocyclic
b. Homocyclic d. Alicyclic
2. The formula which represents the simple whole number ratio of different atoms present in one molecule of a compound is known as :
a. Molecular formula c. Condensed formula
b. Empirical formula d. Electron dot formula
3. An organic compound has empirical formula CH2O and molecular weight 90. It’s molecular formula will be :
a. C6H12O6 c. C2H4O2
b. C3H6O3 d. C3H9O6
4. General formula of an alkene is :
a. CnH2n c. CnH2n-2
b. CnH2n+2 d. CnH2n-1
5. A hydrocarbon is found to contain 81.80% carbon and 18.20% hydrogen. It’s empirical formula will be :
a. C4H8 c. C3H8
b. C2H6 d. C3H6
6. If two compounds have same empirical formula but different molecular formula, they must have :
a. Different percentage composition
b. Different molecular weight
c. Same viscosity
d. Same vapour density
7. Which of the following is an aromatic compound :
a. Benzene hexachloride c. Cyclobutane
b. Cyclohexane d. Toluene
8. Which of the following is a heterocyclic aromatic compound :
a. Nephthalene c. Furan
b. Benzene hexachloride d. Toluene
9. Which of the following belongs to a homologous series ?
a. Methanol, Ethanol, ethanoic acid
b. Propane, Propene, propyne
c. Butane, 2-methylpropane, 2-methylbutane
d. Chloroathane, 2-chloropropane, 1-chlorobutane
10. Which of the following is not true about homologous series ?
a. Adjacent members of group differ by one –CH2 group.
b. Adjacent members of group differs by a mass of 14 amu.
c. Members of a group have same chemical and physical properties.
d. Members of a group can be prepared by same general methods.

a. Isomers c. allotropes
b. Homologues d. None
12. Alkanes are also called :
a. Paraffins c. Acetylene
b. Olefins d. Both ‘a’ and ‘b’
Answer :
1 – d 2 – b 3 – b
4 – a 5 – c 6 – b
7 – d 8 – c 9 – d
10 – c 11 – b 12 – a
References
- Sthapit, M.K., Pradhananga, R.R., Foundations of Chemistry, Vol 1 and 2, Fourth edition, Taleju Prakashan, 2005.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd.,
Hydrocarbons are the organic compounds containing carbon and hydrogen only. Hydrocarbons are broadly classified as – aliphatic, alicyclic and aromatic hydrocarbons. In this note, we are going to study about preparation and properties of aliphalic hydrocarbons i.e open chain hydrocarbons. Aliphatic hydrocarbons are further classified as – saturated hydrocarbons (alkanes) and unsaturated hydrocarbons (alkenes and alkynes).
Contents [hide]
Alkanes (saturated hydrocarbons)
Preparation of alkanes
1. From haloalkanes
a. By Wurtz reaction: When an alkyl halide (haloalkane) is heated with sodium metal in presence of dry ether, an alkane containing double number of carbon atoms than in haloalkane is formed. This reaction is called Wurtz reaction.

Q) Identify A.

b. By reduction: Alkyl halides when reduced with Zn/HCl , H2/Ni, etc. give alkanes. Eg.

2. By catalytic hydrogenation of alkenes and alkynes:
Hydrogenation of unsaturated hydrocarbons (i.e. alkenes and alkynes) in presence of nickel or platinum as catalyst results in the formation of alkanes. Eg.

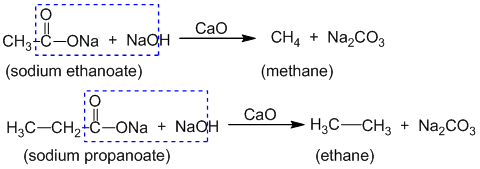
3. By decarboxylation of (salt of) carboxylic acid:
When sodium salt of carboxylic acid is heated with soda-lime (NaOH+CaO), a molecule of carbondioxide is eliminated from the molecule to give alkane. This reaction is called decarboxylation reaction. Eg.
Chemical properties of alkanes
1. Halogenation: This reaction involves the substitution of hydrogen atoms of alkanes by halogen atoms. For example, chlorine reacts with methane in presence of sunlight or heat to form four different halogen derivatives.

2. Nitration: Alkane reacts with nitric acid at high temperature to form nitroalkane. Eg.

3. Sulphonation : When alkane is heated with fuming sulphuric acid, alkane sulphonic acid is formed. Eg.

4. Oxidation: If burnt in air (Oxygen), alkanes are completely oxidized to carbon dioxide and water with large amount of heat.

When burnt in insufficient supply of oxygen, alkane forms carbon monoxide and carbon (carbon black). Eg.

Lower alkanes when heated with limited supply of air at 350-5000C form aldehydes. Eg.

Alkenes
Preparation of alkenes
1. By dehydrohalogenation of alkyl halide ( elimination reaction):
When alkyl halide is heated with alcoholic solution of sodium or potassium hydroxide, hydrogen and halogen atom is eliminated from adjacent carbon atoms to give alkene. Eg.

If there is possibility of the formation of two alkenes, major product is formed according to Saytzeff’s rule. This rule states that when there is a chance of formation of more than one alkene, then the more substituted alkene is formed as major product.
Eg. In the dehydrohalogenation of 2-bromobutane, but-2-ene is the major product over but-1-ene.

2. By dehydration of alcohols:
Removal of water molecule from a molecule is called dehydration. Alcohol undergoes dehydration to form alkene when it is heated with dehydrating agent like sulphuric acid(H2SO4), phosphoric acid(H3PO4), alumina(Al2O3) etc. eg.

If there is possibility of the formation of two alkenes, major product is formed according to Saytzeff’s rule. eg.

3. By controlled hydrogenation of alkynes:
When alkyne is treated with hydrogen in presence of catalyst Pd on BaSO4 poisoned by quinoline, alkene is formed. Eg.

Note: Lindlar’s catalyst = Pd+BaSO4+quinoline
Chemical properties of alkenes
1. Addition of hydrogen (Catalytic hydrogenation):
When alkenes are heated with hydrogen gas in presence of metal catalyst like Ni, Pt or Pd, alkanes are formed. This reaction is called catalytic hydrogenation.

2. Addition of halogens:
Halogens react with alkene in presence of inert solvent like carbon tetrachloride to give dihaloalkane.
Eg. ethene reacts with Br2 in presence of CCl4 to give 1,2-dibromoethane. In this reaction reddish brown colour of bromine is discharged. Hence this is a test reaction of ethene (alkene).

3. Addition of hydrogen halides ( halogen acids)(HCl, HBr, HI):
Alkene reacts with halogen acids to give alkyl halide (haloalkane). Eg.

When alkene is unsymmetrical then the addition takes place according to Markovnikov’s rule.
Markovnikov’s rule:
This rule states that when an unsymmetrical reagent is added to an unsymmetrical alkene, the negative part of the reagent goes to that double bonded carbon which has lesser number of hydrogen atoms.
For example: The addition of HBr to propene gives 2- bromopropane instead of 1- bromopropane.
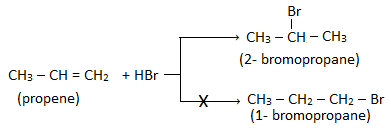
Other example:

Peroxide effect or anti-Markovnikov’s rule:
When HBr is added to an unsymmetrical alkene in presence of organic peroxide, bromine goes to the double bonded carbon atom having more number of hydrogen. This phenomenon of anti- Markovnikov’s addition of HBr caused by the presence of peroxide is known as peroxide effect or anti- Markovnikov’s rule.

4. Addition of water [Catalytic hydration]:
Alkenes react with water in presence of dilute mineral acid as catalyst to form alcohol. Eg.

5. Addition of sulphuric acid:
Alkenes react with concentrated sulphuric acid to give alkyl hydrogen sulphate. Eg.

6. Ozonolysis:
Alkene reacts with ozone to give ozonide. On warming ozonide with Zn in water, it breaks down to give two molecules of carbonyl compounds (aldehyde or ketone). This process of formation of ozonide and it’s decomposition to give carbonyl compounds is called ozonolysis.

7. Polymerization:
The process of making polymers from monomers is known as polymerization. Smaller molecules undergoing polymerization are called monomers. The polymers are high molecular weight large molecules made by the polymerization of monomers.
Ethene polymerizes to form polyethene.

Alkynes
Preparation of alkynes
1. By direct combination of elements (i.e. carbon and hydrogen):
Ethyne (acetylene) gas is formed when an electric spark is struck between two carbon electrodes in an atmosphere of hydrogen gas.

2. By dehydrohalogenation of vicinal dihalides:
When vicinal dihalides are treated with alcoholic KOH, alkynes are formed by dehydrohalogenation. Eg.

Note: Vicinal dihalide– Compounds that contain two hydrogen atoms on adjacent carbon atoms.
3. By heating trihalogen derivatives with silver powder:
Trihaloalkanes like chloroform and iodoform when heated with silver powder form alkynes. Eg.

Chemical properties of alkynes
1. Addition of hydrogen ( Reduction):
When alkyne is heated with hydrogen in presence of Ni, Pt or Pd catalyst, alkane is formed. Eg.

However, alkyne reacts with hydrogen in presence of palladium catalyst deposited over barium sulphate poisoned by sulphur to give alkene. Eg.

2. Addition of halogen acids(HX):
Alkynes react with two molecules of halogen acids according to Markovnikov’s rule to give dihaloalkane. Eg.

3. Addition of water : Catalytic hydration:
Alkynes react with water in presence of mercuric sulphate and sulphuric acid to give vinyl alcohol which rearranges to give aldehyde or ketone.

For example, ethyne gives ethanal (i.e. aldehyde).

Propyne gives propanone (i.e. ketone).

4. Reaction with bromine solution:
Alkynes react with bromine in water or CCl4 to give tetrabromo compound. Here, red colour of bromine is discharged. This is test reaction of alkyne (unsaturated compound).

5. Polymerization reaction:
When alkynes are passed through a red hot iron or copper tube, they polymerize to form aromatic compounds.
Eg. Three molecules of ethyne (acetylene) polymerize to give benzene.

6. Formation of acetylides (Acidic nature of acetylene):
Acetylene is acidic in nature because it releases H+ easily.
a. Action with sodium metal:
Acetylene reacts with molten Na metal to form sodium acetylide.

b. Action with ammonical silver nitrate solution (Tollen’s reagent):
Acetylene reacts with ammonical silver nitrate solution ( i.e. Tollen’s reagent) to give silver acetylide which have white ppt.

c. Action with ammonical cuprous chloride solution:
Acetylene reacts with ammonical cuprous chloride solution to form copper acetylide which have red ppt.


Test of unsaturation ( i.e Test of alkenes and alkynes)
1. Bromine decolorization test:
Red colour of bromine is discharged when Br2 solution in water or carbon tetrachloride is added to unsaturated compounds (alkenes or alkynes). Therefore, this reaction is used to detect the presence of multiple bond in a molecule. Eg.


2. Baeyer’s test ( Oxidation with alkaline solution of KMnO4):
Alkaline solution of potassium permanganate is known as Baeyer’s reagent.
Alkene reacts with Baeyer’s reagent to form glycol, where pink colour of the potassium permanganate is discharged. Therefore this reaction is used as test reaction of alkenes.

Similarly, alkynes also discharge the pink colour of Baeyer’s reagent.
* Baeyer’s reagent oxidizes ethyne to oxalic acid and the pink colour of KMnO4 is discharged.

* Other alkynes react with Baeyer’s reagent to give two molecules of carboxylic acids. Eg.

Q) Give a suitable test to distinguish following pairs.
a. ethyne and ethane
b. ethene and ethyne.
Kolbe’s electrolysis method for the preparation of Alkanes, alkenes and Alkynes
Alkanes, alkenes and alkynes are prepared by electrolysis of salt of monocarboxylic acid, dicarboxylic acid and unsaturated dicarboxylic acid respectively.
1. Preparation of alkanes:
An alkane is obtained by the electrolysis of sodium or potassium salt of a carboxylic acid in aqueous solution. Eg.
Ethane is produced at anode during the electrolysis of an aqueous solution of sodium or potassium acetate as follows:

2. Preparation of alkenes:
An alkene is obtained by the electrolysis of sodium or potassium salt of a dicarboxylic acid in aqueous solution. Eg.
Ethene is produced at anode during the electrolysis of an aqueous solution of sodium or potassium succinate as follows:

3. Preparation of alkynes:
An alkyne is obtained by the electrolysis of sodium or potassium salt of an unsaturated dicarboxylic acid in aqueous solution. Eg.
Ethyne is produced at anode during the electrolysis of an aqueous solution of sodium or potassium maleate (i.e. salt of maleic acid) as follows:
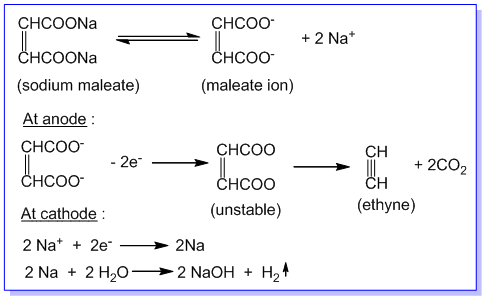
References
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Sharma, M.L., Chaudary, P.N., A Text Book of B.Sc. Chemistry, Second edition (Volume one and two), Ekta Books, Kathmandu, 2004.
- Sthapit, M.K., Pradhananga, R.R., Foundations of Chemistry, Vol 1 and 2, Fourth edition, Taleju Prakashan, 2005.
- https://en.wikipedia.org/wiki/Acetylene
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Map%3A_Organic_Chemistry_(Smith)/Chapter_01%3A_Structure_and_Bonding/1.9%3A_Ethane%2C_Ethylene%2C_and_Acetylene
- https://www.britannica.com/science/hydrocarbon/Nomenclature-of-alkenes-and-alkynes.
- Kolkatta, 2007.
- https://socratic.org/organic-chemistry-1/ways-to-draw-and-represent-molecules/condensed-structure
- https://www.britannica.com/science/homologous-series
Witting reaction: Examples and Mechanism
Contents [hide]
What is witting reaction?
Triphenyl phosphonium alkylide (simply phosphorous ylide) is called witting reagent. When carbonyl compound (aldehyde or ketone) is treated with an ylide, olefin (alkene) is formed. This reaction is called witting reaction.
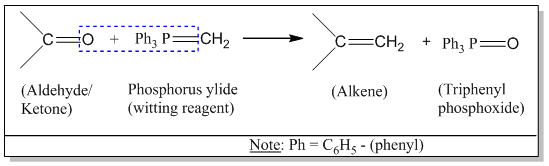
Mechanism of witting reaction
Question- Answer from witting reaction
Q) Give the reaction and mechanism when 2-hexanone is treated with witting reagent.
When 2-hexanone is treated with witting reagent, 2-methylhex-1-ene is formed. This reaction is called witting reaction. The reaction involved and mechanism is given below.

Mechanism:
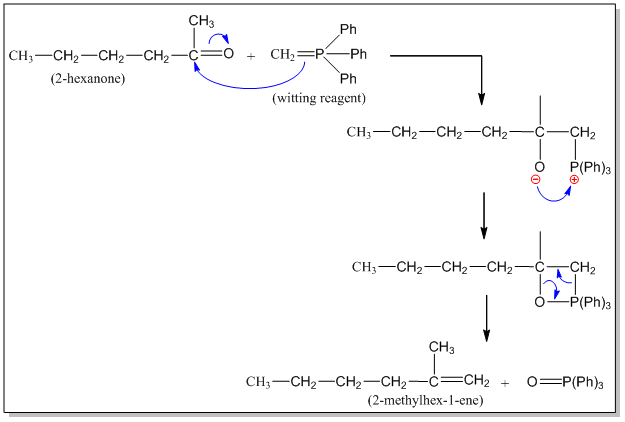
Q) Give two different methods for witting synthesis of 2-methyl-1-hexene.

2-methyl-1-hexene can be prepared using witting synthesis by following two methods:
Method-I :

Method-II :

Q) Write product and mechanism of the reaction:

This reaction is witting reaction. The reaction involved and mechanism is given below:

Mechanism:

References
- Morrison, R.T. , Boyd, R.N., Organic Chemistry, Sixth edition, Prentice-Hall of India Pvt. Ltd., 2008.
- March, j., Advanced Organic Chemistry, Fourth edition, Wiley Eastern Ltd. India, 2005.
- https://chemicalnote.com/category/organic-chemistry/name-reactions/
IUPAC Nomenclature of Organic Compounds :
Contents [hide]
- IUPAC Nomenclature of organic compounds containing carbon to carbon single bonds and substituents only : [A]
- IUPAC Nomenclature of organic compounds containing multiple bonds(double/triple bond) too. [B]
- IUPAC Nomenclature of organic compounds containing one functional group ( monofunctional compounds): [C]
- IUPAC Nomenclature of organic compounds containing more than one functional groups ( polyfunctional compounds) : [D]
- CONCLUSION :
- References :
What is IUPAC nomenclature?
This is a method of naming the organic compounds as recommended by the international Union of Pure and Applied Chemistry (IUPAC).
Each part of the IUPAC name gives you some useful information about the compound.
Before starting the IUPAC rules, lets see an example of organic compound and it’s IUPAC name.
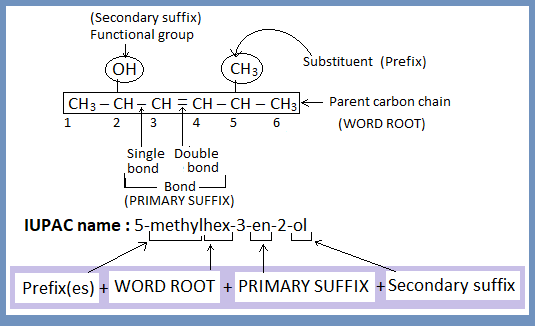
To understand the name you need to take the name to pieces. In the given example “5-methylhex-3-en-2-ol” there are 4 pieces- ‘methyl’, ‘hex’, ‘en’ and ‘ol’.
‘methyl’ tells that –CH3 is present as substituent.
‘hex’ tells that there are 6 carbon atoms on parent carbon chain.
‘en’ tells that there is at least one carbon to carbon double bond.
‘ol’ tells that there is –OH group(alcohol) as functional group.
Thus, general format for IUPAC name of all compounds is:

IUPAC name of all compounds contain word root and primary suffix but prefix and secondary suffix may not be present because all organic compounds must contain carbon chain and bond but substituent and functional group may not be present. For example.
Now see the four parts ( prefix, word root, bond and functional group) separately.
1. Word root : It indicates the parent carbon chain, which is the the longest continuous chain of carbon atoms including functional group and multiple bonds( if present).
| No. of Carbon atoms in the parent chain | Word root |
| 1 | Meth- |
| 2 | Eth- |
| 3 | Prop- |
| 4 | But- |
| 5 | Pent- |
| 6 | Hex- |
| 7 | Hept- |
| 8 | Oct- |
| 9 | Non- |
| 10 | Dec- |
| 11 | Undec- |
| 12 | Dodec- |
2. Primary suffix : It indicates the nature of carbon to carbon bond in the parent carbon chain.
| Nature of carbon to carbon bond | Primary suffix |
| Single | -ane |
| Double | -ene |
| Triple | -yne |
3. Secondary suffix : It indicates the parent(main) functional group present in the compound.
| S. N. | Class or family | General structural formula | Functional group | Secondary suffix |
| 1 | Carboxylic acid |  Or RCOOH |  Or -COOH | -oic acid |
| 2 | Sulphonic acid (sulfonic acid) | R-SO3H | -SO3H | -sulphonic acid |
| 3 | Acid anhydride |  |  | -oic anhydride |
| 4 | Ester |  Or RCOOR |  – COOR | -oate |
| 5 | Acid chloride |  Or RCOCl |  -COCl | -oyl chloride |
| 6 | Acid amide |  Or RCONH2 |  Or -CONH2 | -amide |
| 7 | Nitrile |  Or RCN | -CN | -nitrile |
| 8 | Aldehyde |  Or RCHO |  Or -CHO | -al |
| 9 | Ketone |  |  | -one |
| 10 | Alcohol | R-OH | -OH | -ol |
| 11 | Thioalcohol | R-SH | -SH | -thiol |
| 12 | Amine | R – NH2 | -NH2 | -amine |
4. Prefix : It indicates the substituent ( i.e any group bonded with parent carbon chain except main functional group).
| Substituent | Prefix |
| -CH3 | Methyl- |
| -CH2CH3 or–C2H5 | Ethyl- |
| -OCH3 | Methoxy- |
| -OCH2CH3 or–OC2H5 | Ethoxy- |
| -F | Fluoro- |
| -Cl | Chloro- |
| -Br | Bromo- |
| -I | Iodo- |
| -NO2 | Nitro- |
| -NO | Nitroso- |
| -NH2 | Amino- |
{Note: -NH2 can be taken as substituent as well as functional group}
Sometimes, in case of compounds having polyfunctional groups, functional groups may be considered as prefixes. For example:
| -OH | Hydroxy- |
| -CN | Cyano- |
| -CO- | Keto- (Carbonyl or Oxo-) |
| -CHO | Aldo- (Carbonyl or Oxo-) |
General steps for IUPAC nomenclature of organic compounds:
Naming of all organic compounds can be done in three steps as,
To simplify the nomenclature process, differentiate the organic compounds in four categories as,
[A] Compounds containing carbon to carbon single bonds and substituents only.
[B] Compounds containing multiple bonds ( double/ triple bonds) too.
[C] Compounds containing one functional group ( monofunctional compounds).
[D] Compounds containing more than one functional groups ( polyfunctional compounds).
IUPAC Nomenclature of organic compounds containing carbon to carbon single bonds and substituents only : [A]
There are only three steps in nomenclature of all organic compounds.
Step-I : Selection of parent chain : The longest continuous carbon chain is selected as the parent chain. Eg.


If two or more equally long chains are present, the chain with maximum number of substituent is selected as the parent chain.

Step-II : Numbering the parent chain :
Lowest locant rule: Carbon bearing the substituent gets the lowest possible locant. Locant is a number that locate the position of substituent.


Note : Here the first locant is same (i.e 2) so compare second locant which is 2 and 4 in (2,2,4) and (2,4,4). Hence (2,2,4) is lowest set of numbering and is correct numbering.
If substituents are present at equivalent position, follow alphabetical order.


Step III – Naming : Alphabetically i.e. if more than one substituents are present then they are written in the alphabetical order.






Note: Naming of compounds containing complex substituent (alkyl group) :An alkyl substituent having substituents within itself is named in bracket by putting number outside. Eg.
IUPAC Nomenclature of organic compounds containing multiple bonds(double/triple bond) too. [B]
Step-I : Selection of parent chain : The longest continuous carbon chain including multiple bond is selected as the parent chain. Eg.


Step-II : Numbering the parent chain :
Lowest locant rule: Carbon bearing the multiple bond gets the lowest possible locant.

If both substituent and multiple bond are present, the priority order is:
Multiple bond > Substituent
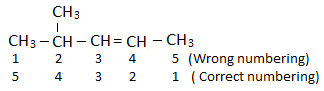


If multiple bonds are present at equivalent position, follow alphabetical order i.e double bond(-ene) gets higher priority than triple bond(-yne).

Step III – Naming : Alphabetically i.e. if more than one substituents and multiple bonds are present then they are written in the alphabetical order.




Note: If both double and triple bonds are present, the terminal ‘e’ of first one(in name) is dropped(removed).
IUPAC Nomenclature of organic compounds containing one functional group ( monofunctional compounds): [C]
Step-I : Selection of parent chain : The longest continuous carbon chain including functional group is selected as the parent chain. Eg.

Step-II : Numbering the parent chain : Now the final priority order for numbering is :
Functional group > Multiple bond > substituent


Step III – Naming : Always follow this format:

For example,


NOTE : While adding the secondary suffix to the primary suffix, the terminal ‘e’ of the primary suffix (i.e., ane, ene or yne) is dropped if the secondary suffix begins with a vowel. However, the terminal ‘e’ is retained if the complete secondary suffix begins with a consonant.
Some examples of IUPAC names for compounds containing one functional group:
| Class or family | General formula | Examples |
| Carboxylic acid |  Or RCOOH |  |
| Sulphonic acid (sulfonic acid) | R-SO3H |  |
| Ester |  Or RCOOR |  |
| Acid chloride |  Or RCOCl |  |
| Acid amide |  Or RCONH2 |  |
| Nitrile |  Or RCN |  |
| Aldehyde |  Or RCHO |  |
| Ketone |  |  |
| Alcohol | R-OH |  |
| Amine | R – NH2 |  |
IUPAC Nomenclature of organic compounds containing more than one functional groups ( polyfunctional compounds) : [D]
The priority order of functional groups is:

-COOH > -SO3H > – COO- > -COX > -CONH2 > -CN > -CHO > -CO- > -OH > -NH2
During nomenclature of polyfunctional compounds, fuctional group of higher priority is taken as principal functional group and other functional groups are considered as substituents.
The IUPAC rules applied for monofunctional compounds are also applied for polyfunctional compounds. Some additional rules are needed, which are given below:
At first, principal functional group is identified according to priority order. Eg.

Step-I : Selection of parent chain : The longest continuous carbon chain with principal functional group is selected as the parent chain. Eg.

Step-II : Numbering the parent chain : Principal functional group gets lowest locant(number).

Step III – Naming : Substituents are written in alphabetical order as mentioned earlier. Eg.

Some other examples of polyfunctional compounds :





- If a compound contains two or more functional groups, the words –di, -tri, -tetra, etc. is written before the name of secondary suffix with respective locant numbers. In such cases, the last letter ‘e’ from primary suffix is not dropped (removed). Eg.
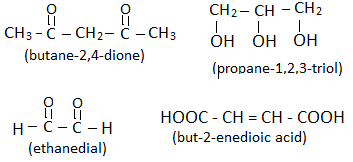
CONCLUSION :
Nomenclature of organic compounds is very easy. Remember only two things (mentioned below) during nomenclature, you will easily write correct IUPAC name of all organic compounds.
1. Always follow three steps :

2. Always write the name in general format :

References :
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- https://iupac.org/what-we-do/nomenclature/
- https://app.biorender.com/illustrations/edit/5ed85ef66c525700aadc31bf
- https://en.wikipedia.org/wiki/Preferred_IUPAC_name
Aldehydes and Ketones – Carbonyl compounds – Preparation and Properties
Contents [hide]
- Carbonyl compounds [Aldehydes and ketones]
- Structure and nature of the carbonyl group
- Nomenclature of aldehydes and ketones
- Isomerism in aldehydes and ketones
- General methods of preparation of aldehydes and ketones
- Physical Properties of aldehydes and ketones
- [A] Nucleophilic addition reaction
- [B] Addition followed by elimination of water molecule [Addition of ammonia derivatives]
- 2,4-DNP test
- [C] Oxidation reactions of aldehydes
- [D] Haloform reaction
- [E] Reduction reaction
- [F] Special reactions of methanal (formaldehyde)
- Aldol condensation reaction
- Cannizzaro’s reaction
- Formalin and its Uses
- REFERENCES
Carbonyl compounds [Aldehydes and ketones]
Aldehydes and ketones are the compounds containing carbonyl group, so are collectively called carbonyl compounds.

Structure and nature of the carbonyl group
In carbonyl group, there is carbon to oxygen double bond, which consist of a sigma (σ) bond and a pi (π) bond
 .
.
Both of the carbon and oxygen atoms are sp2 hybridized. One sp2 hybrid orbital of carbon forms σ-bond with oxygen atom and remaining two hybrid orbitals form σ-bond with hydrogen or carbon atom. π -bond is formed by the overlap of unhybridized orbitals of carbon and oxygen atom.
This carbonyl group is trigonal and planar with bond angle of 1200.

In carbonyl group, carbon atom is bonded with oxygen atom which is more electronegative than carbon. Thus, the bonded pair of electrons lie more closer to the oxygen atom than carbon atom which leads to the polarization in carbon-oxygen bond. There is charge separation, oxygen atom acquires slightly negative charge while the carbon atom acquires slightly positive charge.

Nomenclature of aldehydes and ketones

Isomerism in aldehydes and ketones
1 . Chain Isomerism: Aldehydes having at least 4 carbon atoms and ketones having at least 5 carbon atoms show chain isomerism. Eg.

2. Position isomerism: Ketones having at least 5 carbon atoms and aromatic aldehydes show position isomerism.Eg.

3. Functional isomerism: Ketone and aldehyde having same molecular formula are functional isomers of one another.

4. Metamerism: Ketones exhibit metamerism due to difference in alkyl group present on either side of carbonyl group.
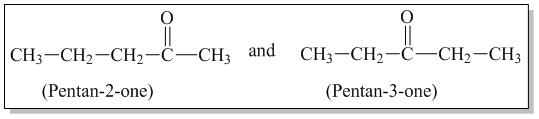
Q) Write the possible isomeric aldehydes and ketones that can be formed from C4H8O.

General methods of preparation of aldehydes and ketones
1. From alcohol:
(i) By oxidation of alcohols:
- Alcohols on controlled oxidation give aldehydes and ketones.
- Acidified KMnO4 or K2Cr2O7 is used as oxidizing agent.
- 10 alcohol gives aldehyde while 20 alcohol gives ketone. Eg.

(ii) By dehydrogenation of alcohols:
When alcohol vapors are passed over heated copper at 3000C, different types of alcohols give different products.
- Primary alcohols are dehydrogenated to aldehydes. Eg.

- Secondary alcohols are dehydrogenated to ketones. Eg.

2. By ozonolysis of alkene:
Alkene reacts with ozone to give ozonide. On warming ozonide with Zn in water, it breaks down to give two molecules of carbonyl compounds (aldehyde or ketone). This process of formation of ozonide and it’s decomposition to give carbonyl compounds is called ozonolysis.
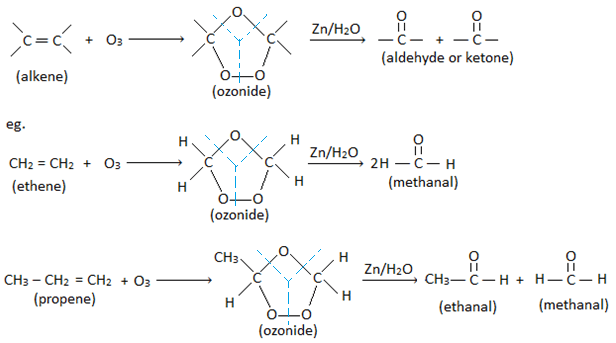
3. By catalytic hydration of alkynes :
Alkynes react with water in presence of mercuric sulphate and sulphuric acid to give vinyl alcohol which rearranges to give aldehyde or ketone.

For example, ethyne gives ethanal (i.e. aldehyde).

Propyne gives propanone (i.e. ketone).

4. From acid chlorides:
(i) By Rosenmund reduction: Aldehydes can be prepared by reducing acid chloride solution with hydrogen in the presence of Palladium(Pd) catalyst deposited on barium sulphate and partially poisoned with sulphur or quinoline. This reaction is called Rosenmund reduction.

(ii) Ketones can be prepared by treating acid chloride with dialkyl cadmium.

Q) How would you convert benzoic acid into benzaldehyde?

5. From gem-dihalides:
The alkaline hydrolysis of gem-dihalide gives aldehyde and ketone.
Aldehydes are formed when two halogen atoms are attached to terminal carbon atom.
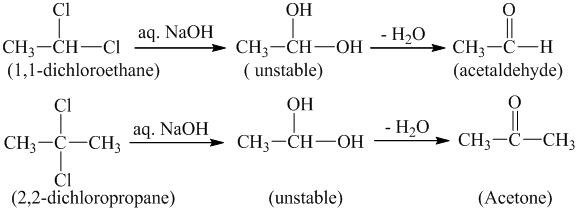
Ketones are formed when two halogen atoms are attached to non-terminal carbon atom.
Physical Properties of aldehydes and ketones
1. Boiling point: Aldehydes and ketones have higher boiling point than hydrocarbon of comparable molecular masses. This is because aldehydes and ketones contain polar carbonyl group and therefore there exists strong dipole-dipole interaction between the opposite end of C=O dipoles.

However, aldehydes and ketones have lower boiling point than alcohols and carboxylic acid of comparable molecular masses. This is because dipole-dipole interaction is weaker than intermolecular H-bonding.
2. Solubility: Lower aldehydes and ketones containing up to 4 carbon atoms are soluble in water due to formation of hydrogen bond between the polar carbonyl group and water molecule.

Chemical Properties
[A] Nucleophilic addition reaction
Aldehydes and ketones undergo nucleophilic addition reaction due to presence of polar carbonyl group.

The positively charged carbon of carbonyl group is readily attacked by nucleophilic species for the initiation of reaction. This leads to the formation of intermediate anion which is then attacked by electrophile (eg.H+) to give the final addition product.
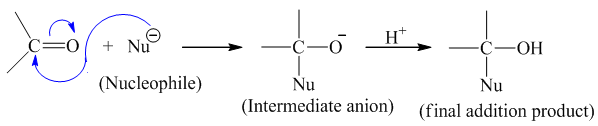
Q) Why does aldehydes easily undergoes nucleophilic addition reaction as compared to that of ketone?
Aldehydes and ketones contain polar carbonyl group and hence carbonyl carbonyl carbon is a suitable site for nucleophilic attack.

In aldehydes, one electron releasing alkyl group is attached to carbonyl carbon while in ketone two alkyl groups are attached to carbonyl carbon. Thus, aldehydic carbon is electron deficient than ketonic carbon and hence aldehyde is more easily attacked by nucleophilic species.
1. Addition of HCN: Aldehydes and ketones react with hydrogen cyanide to form addition product called cyanohydrins. Eg.

Cyanohydrin on acidic hydrolysis gives α-hydroxy acids which on heating loses a molecule of water to form α,β-unsaturated acid.

Q) Identify A, B , C and D.

Q) How would you obtain 2-hydroxy-2-methylpropanoic acid from propanone?
2. Addition of sodium bisulphite: Aldehydes and ketones react with saturated solution of sodium bisulphite to form crystalline bisulphite addition products. Eg.

3. Addition of Grignard reagent:
Aldehydes and ketones (i.e carbonyl compounds) when treated with Grignard reagent gives addition product, which on acidic hydrolysis give alcohols.

⊗ Formaldehyde gives primary alcohol. Eg.

⊗ Aldehydes other than formaldehyde give secondary alcohol. Eg.

⊗ Ketones give tertiary alcohol. Eg.

[B] Addition followed by elimination of water molecule [Addition of ammonia derivatives]
Aldehydes and ketones react with number of ammonia derivatives such as hydroxylamione(NH2OH), hydrazine(NH2-NH2), phenylhydrazine(C6H5NHNH2), etc. in weakly acidic medium to form compounds containing C=N group.
1. Reaction with hydroxylamine: Aldehydes and ketones react with hydroxylamine to form oximes. Eg.
![Addition followed by elimination of water molecule [Addition of ammonia derivatives]](https://chemicalnote.com/wp-content/uploads/2022/02/word-image-32.png)
2. Reaction with hydrazine: Aldehydes and ketones react with hydrazine to form hydrazone. Eg.

3. Reaction with phenyl hydrazine: Aldehydes and ketones react with phenyl hydrazine to form phenylhydrazones. Eg.
![Addition followed by elimination of water molecule [Addition of ammonia derivatives]](https://chemicalnote.com/wp-content/uploads/2022/02/word-image-34.png)
4. Reaction with 2,4-dinitrophenyl hydrazine : Aldehydes and ketones react with 2,4-dinitrophenyl hydrazine (2,4-DNP) to form yellow, orange or red ppt. of 2,4-dinitrophenyl hydrazone. Eg.

5. Reaction with semicarbazide: Aldehydes and ketones react with semicarbazide to form semicarbazone. Eg.
![Addition followed by elimination of water molecule [Addition of ammonia derivatives]](https://chemicalnote.com/wp-content/uploads/2022/02/word-image-36.png)
2,4-DNP test
Aldehydes and ketones react with 2,4-dinitrophenyl hydrazine (2,4-DNP) to form yellow, orange or red ppt. of 2,4-dinitrophenyl hydrazone. Eg.

Q) Write a chemical test to distinguish ethanal from ethanol?
Ethanal and ethanol can be distinguished by 2,4-DNP test. Ethanal (i.e aldehyde) reacts with 2,4-dinitrophenyl hydrazine (2,4-DNP) to form orange ppt. of ethanal 2,4-dinitrophenyl hydrazone. But ethanol does not give this test.

Note : 2,4-DNP = Brady’s reagent.
≠ Action with PCl5:
Aldehydes and ketones react with PCl5 to give gem-dichloroalkane (gem-dihalide).

[C] Oxidation reactions of aldehydes
Aldehydes are oxidized not only by strong oxidizing agents like KMnO4 or K2Cr2O7 but also by weak oxidizing agents like Br2 water, Ag+, Cu++, etc. So, aldehydes are strong reducing agents.
1. Reaction with Tollen’s reagent: [Silver mirror test]
Tollen’s reagent is an ammonical solution of silver nitrate. It is prepared by adding dilute solution of NH4OH to AgNO3 solution till the precipitate of Ag2O once formed gets dissolved.

Aldehydes on heating with Tollen’s reagent reduces the reagent to metallic silver.
![Reaction with Tollen’s reagent: [Silver mirror test]](https://chemicalnote.com/wp-content/uploads/2022/02/word-image-41.png)
The silver deposits on the inner wall of test tube forming a shining layer like mirror. Hence, this test is known as silver mirror test.
- Both aliphatic and aromatic aldehydes give this test but ketones do not give this test.
Q) Write the functional isomer of C3H6O and give a chemical test to distinguish them.
The functional isomers of C3H6O are:

These two isomers can be distinguished by silver mirror test (i.e. Tollen’s reagent). Propanal (i.e. aldehyde) gives positive silver mirror test but propanone (i.e. ketone) does not give this test.

2. Reaction with Fehling’s solution: [Fehling’s test]
Fehling’s solution is an alkaline solution of CuSO4 containing some Rochelle salt (i.e. sodium potassium tartarate. It is prepared by adding alkaline solution of Rochelle salt [Fehling solution B] to CuSO4 solution [Fehling solution A]. When an aliphatic aldehyde is heated with Fehling’s solution, a brick red ppt. of cuprous oxide is formed. This reaction is known as Fehling’s test.
![Reaction with Fehling’s solution: [Fehling’s test]](https://chemicalnote.com/wp-content/uploads/2022/02/word-image-44.png)
[D] Haloform reaction
Aldehydes and ketones containing CH3CO- group on reaction with excess halogen in presence of NaOH gives haloform (chloroform’ bromoform, iodoform). Eg.

Iodoform test :

Note:
- This reaction occurs in same way as lab preparation of chloroform. Eg.

- This reaction is used to distinguish some of the pairs of compounds. Eg.
- Ethanol and methanol
- Ethanal and methanal
- 2-pentanone and 3-pentanone, etc.
Q) How can you distinguish 2-pentanone and 3-pentanone.
2-pentanone and 3-pentanone can be distinguished by iodoform test as 2-pentanone gives iodoform reaction but 3-pentanone doesn’t give iodoform reaction. Eg.

[E] Reduction reaction
1. Reduction to alcohols: Aldehydes and ketones are reduced to primary and secondary alcohols respectively using H2 in presence of Ni, Pt, Pd or LiAlH4. Eg.


2 . Clemmensen’s reduction: The reduction of aldehydes and ketones to alkane using zinc amalgam and conc. HCl is Clemmensen’s reduction. In this reaction, carbonyl group (-CO-) is reduced to methylene group (-CH2-). Eg.

3 . Wolff-Kishner reduction : In this method aldehyde and ketone is treated with hydrazine to form hydrazone which is then heated with KOH in presence of glycol to give alkane. Eg.

4 . Reduction with HI in presence of red P : Aldehydes and ketones can be reduced into corresponding hydrocarbon when heated with HI in presence of red phosphorus at 1500C.

[F] Special reactions of methanal (formaldehyde)
1. Reaction with ammonia: Formaldehyde reacts with ammonia to form hexamethylene tetramine which is commonly known as ‘urotropine’. It is used as medicine to treat urinary infections.

2. Reaction with phenol:
Phenol condenses with formaldehyde in the presence of an acid or basic catalyst to form a polymer called Bakelite.

Formation of linear polymer:

Formation of cross-linked polymers:

Aldol condensation reaction
Condensation between two molecules of aldehydes or ketones having at least one α – hydrogen atom in presence of dilute alkali to form β-hydroxy aldehyde or β-hydroxy ketone is known as aldol condensation reaction. Examples:

Aldehydes and ketones which do not contain any α – hydrogen atom such as HCHO, (CH3)3CCHO, C6H5CHO, etc. do not undergo aldol condensation reaction.
Note : Dehydration of aldol product gives α, β-unsaturated aldehyde or ketone.

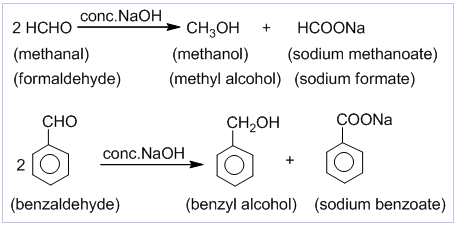
Cannizzaro’s reaction
Aldehydes which do not contain α-hydrogen like HCHO, C6H5CHO,etc. undergo self oxidation and reduction on treatment with conc. alkali. In this reaction one molecule is oxidized to carboxylic acid and other molecule is reduced to alcohol. Thus, a mixture of an alcohol and a salt of carboxylic acid is formed by Cannizzaro’s reaction
Formalin and its Uses
A 37-40% solution of formaldehyde in water is called formalin. Its molecular formula is HCHO (i.e. formaldehyde).
Uses of Formalin:
- It is used in preservation of biological specimens.
- It is used as an antiseptic and disinfectant.
- It is used to manufacture urinary antiseptic i.e. Urotropin.
- It is used to manufacture polymers like Bakelite, resins, etc.
- It is used in the manufacture of dyes like indigo, pararosaniline, etc.
REFERENCES
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Morrison, R.T. , Boyd, R.N., Organic Chemistry, Sixth edition, Prentice-Hall of India Pvt. Ltd., 2008.
- March, j., Advanced Organic Chemistry, Fourth edition, Wiley Eastern Ltd. India, 2005.
- https://chemicalnote.com/category/organic-chemistry/name-reactions/
- https://www.dailymotion.com/video/x3nq0aj
- https://www.rxlist.com/formalin/definition.htm
Haloalkanes – Nomenclature, Isomerism, Preparation and Properties.
Contents [hide]
What are haloalkanes ( alkyl halides) ?
Alkyl halides or haloalkanes are the organic compounds in which halogen atom is bonded to an alkyl group. The general formula of these compounds is R – X .

Classification of haloalkanes
Haloalkanes are classified into primary, secondary and tertiary haloalkanes depending upon the number of carbon atoms to which halogen linked carbon is bonded.
1. Primary haloalkane ( 10 haloalkane) :
A haloalkane in which halogen linked carbon is bonded to none or one other carbon atom is known as primary haloalkane.
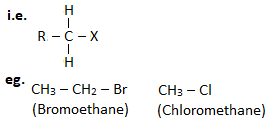
2. Secondary haloalkane ( 20 haloalkane) :
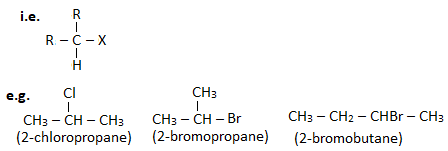
A haloalkane in which halogen linked carbon is further bonded to two other carbon atoms is known as secondary haloalkane.
3. Tertiary haloalkane ( 30 haloalkane) :
A haloalkane in which halogen linked carbon is further bonded to three other carbon atoms is known as tertiary haloalkane.
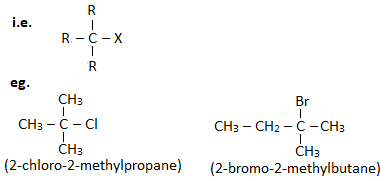
Nomenclature of haloalkanes
A. Common system :
- In common system, haloalkanes are named by adding the word halide after the name of alkyl group.
- The words n-, sec-, tert-, iso- and neo- are usually used in writing the common names.
Common name of alkyl halide is always written as two separate words.
B. IUPAC system :
- In IUPAC system, haloalkanes are named by adding prefix ‘halo-‘ before the name of parent alkane.
The IUPAC name of any monohalo alkanes is always written as one word.

Note : n = straight chain (10)
sec = 20 , tert = 30
iso = 10 ( if second last carbon contains one methyl group and no other branches)
neo = 10 ( if second last carbon contains two methyl groups and no other branches)
Q) Write the structural formula of sec-pentyl chloride, tert-pentyl chloride and neohexyl chloride. Write their IUPAC name too.
Polyhalogen compounds :
Organic compounds containing more than one halogen atom in their molecules are known as polyhalogen compounds. Eg.

Note: Vicinal dihalide : compounds that have halogens on adjacent carbon atoms.
Isomerism in haloalkanes
Haloalkanes show chain isomerism and position isomerism.
1. Chain isomerism :
Haloalkanes having 4 or more carbon atoms show isomerism in which isomers differ in the nature of carbon chain. Eg.

2. Position isomerism :
Haloalkanes having 3 or more carbon atoms show position isomerism in which isomers differ in the position of halogen atom. Eg.

Q. Write down the possible isomers of molecular formula – (i)C4H9Br (ii)C5H11Br. Give their IUPAC names and also specify them as 10, 20 and 30 haloalkanes.
Ans.

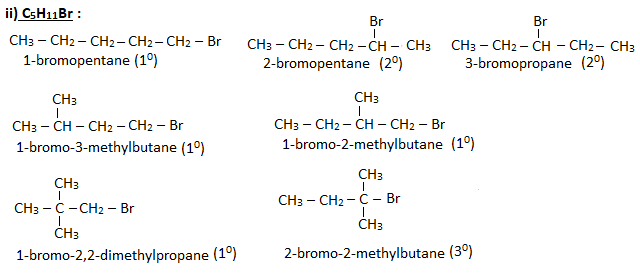
Preparation of haloalkanes
A. Preparation of haloalkanes from alcohols :
1. By the action of halogen acids :
Alcohols can be converted into haloalkanes by treating it with halogen acids in the presence of dehydrating agent such as anhydrous zinc chloride or conc. H2SO4.

Where, HX = HCl, HBr, HI
The reactivity of halogen acids follows the order :
HCl < HBr < HI
# It is because of the fact that bond dissociation energy is : HCl>HBr>HI.
Reactivity of alcohols towards this reaction is :
Primary<Secondary<Tertiary.
# It is because of the fact that greater the number of electron releasing groups on α- carbon atom of alcohol, more is the polarity of C – OH bond. Greater the polarity of C –OH bond, the more reactive is the alcohol.
Examples :

Note : The mixture of HCl and ZnCl2 is known as “Lucas reagent”.

2. By the action of phosphorus halides :
Alcohols on refluxing with phosphorus penta or trihalides give haloalkane.

For example :

Note :
H3PO3 = phosphorous acid
H3PO4 = phosphoric acid
POCl3 = phosphorus oxychloride ( IUPAC = Phosphoryl trichloride )
3. By the action of thionyl chloride :
Alcohols when refluxed with thionyl chloride in presence of pyridine gives chloroalkane.
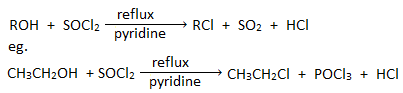
Note : SOBr2 and SOI2 are unstable, therefore bromo and iodo alkanes can not be prepared by this method.
B. Preparation of haloalkanes from hydrocarbons :
1. From alkanes :
- Alkanes when treated with limited amount of halogen in the presence of heat, light or suitable catalyst gives haloalkanes.

- If excess of halogen is used then a mixture of mono and polysubstituted products is obtained.

- Bromination also takes place in similar manner.
- Iodination is reversible thus the reaction is always carried out in the presence of oxidizing agent like HIO3, conc. HNO3, etc. The use of oxidizing agent is to oxidize ‘HI’ formed during the reaction into ‘I2’ and hence shifts the equilibrium in forward direction.

2. From alkenes : By the action of halogen acids (HCl, HBr and HI) :
Alkene reacts with halogen acids to give alkyl halide (haloalkane). Eg.

When alkene is unsymmetrical then the addition takes place according to Markovnikov’s rule.
Markovnikov’s rule :
This rule states that when an unsymmetrical reagent is added to an unsymmetrical alkene, the negative part of the reagent goes to that double bonded carbon which has lesser number of hydrogen atoms.
For example: The addition of HBr to propene gives 2- bromopropane instead of 1- bromopropane.

Peroxide effect :
When HBr is added to an unsymmetrical alkene in presence of organic peroxide, bromine goes to the double bonded carbon atom having more number of hydrogen. This phenomenon of anti- Markovnikov’s addition of HBr caused by the presence of peroxide is known as peroxide effect or anti- Markovnikov’s rule or Kharash effect.

It may be noted that peroxide effect or Kharash effect applies to the addition of HBr only and not to the addition of HCl or HI. This is because H – Cl bond is stronger than H – Br bond due to which cleavage of H – Cl bond to give Cl is unfavorable while in case of H – I the bond is cleaved very easily to give I but the I radical formed immediately combine with each other to form I2 rather than forming C – I bond.
Physical properties of haloalkanes
1.Physical state :
Lower member of alkyl halide are gaseous at room temperature (upto C5) and higher members of alkyl halide are colourless liquid or solid.
2. Boiling point :
- The boiling points of haloalkanes having same alkyl group follows the order :
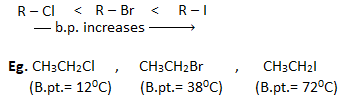
This is because with the increase in size and mass of halogen atom, the magnitude of Vander Waal’s force increases and hence boiling point also increases.
- For the alkyl halides having same halogen atoms boiling point increases with the increase in size of alkyl group. Eg.

- With the increase in branching of alkyl group, surface area decreases and magnitude of Vander Waal’s force also decreases. Hence, boiling point of isomeric alkyl halides decreases with the increase in branching of alkyl group.
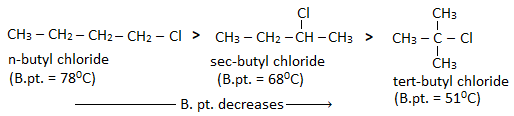
Haloalkanes have higher boiling point than alkane of comparable molecular masses. This is because haloalkanes are polar in nature and due to their polarity, a strong dipole-dipole interaction exist between the molecules of haloalkanes.

3. Solubility :
Although haloalkanes are polar in nature, they are insoluble in water because they are not able to form hydrogen bonds with water molecules. But, they are soluble in organic solvents like benzene, ether, alcohol, etc.
Chemical properties of haloalkanes
- Nucleophilic substitution reaction.
- Elimination reaction
- Reaction with metals
- Reduction reaction
1. Nucleophilic substitution reaction in haloalkanes
A nucleophilic substitution reaction is a chemical reaction which involves the displacement of leaving group by a nucleophile. In this process, the leaving group i.e. the halogen atom departs along with the bonding pair of electrons and the electrons for the formation of the new bond are furnished (provided/supplied) by the nucleophile.

It is of two types :
i. SN1 reaction :
- SN1 indicates the unimolecular nucleophilic substitution reaction.
- The rate of SN1 reaction depends only upon the concentration of the substrate.
i.e. Rate = k[Substrate]
- The reaction occurs in two steps. In first step carbocation is formed and in second step nucleophile attacks the carbocation to give substituted product.
Eg.

- Rate of the reaction is directly proportional to the stability of carbocations. Hence, the order of reactivity is : 30 > 20 > 10 haloalkanes.
ii. SN2 reaction :
- SN2 indicates the bimolecular nucleophilic substitution reaction.
- The rate of SN2 reaction depends upon the concentration of the both substrate and nucleophile.
i.e. Rate = k[Substrate][:Nu –]
- The reaction occurs in single step. . SN2 reaction occurs through a transition state as shown below: Eg.

- Rate of reaction is inversely proportional to the bulkiness of groups attached to the C atom. Hence, the order of reactivity is : 10 > 20 > 3O haloalkanes.
1. Substitution by hydroxyl group ( formation of alcohols) :
When a haloalkane is boiled with aqueous solution of KOH or moist silver oxide (Ag2O), gives alcohol.
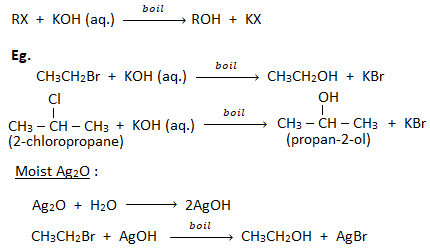
2. Substitution by cyano group ( formation of cyanides or nitriles) :
When haloalkane is treated with alcoholic KCN solution it gives alkane nitrile ( or alkyl cyanide) as the major product.

The alkyl cyanide so produced can be used as the starting material for the preparation of a number of other compounds.
- Partial hydrolysis with conc. HCl or alkaline solution of H2O2 gives amide.

- Complete hydrolysis with dil. HCl gives carboxylic acid.

- Reduction with nascent hydrogen produced by LiAlH4 (Lithium aluminium hydride), Na/alcohol or H2/Ni gives primary amine.


3. Substitution by isocyanide (i.e. – NC ) [ formation of isocyanide] :
Alkyl halide when treated with alcoholic solution of silver cyanide (AgCN) gives alkyl isocyanide.

Alkyl isocyanide on reduction gives secondary amine.


Q) Haloalkane gives alkyl cyanide with KCN while alkyl isocyanide with AgCN, why?
Cyanide ion is an ambient nucleophile which has two sites i.e. carbon and nitrogen through which it can attack alkyl halide. KCN is an ionic compound which ionizes to give cyanide ion (CN–), the negative charge of which attacks the alkyl halide to give alkyl cyanide. But AgCN is a covalent compound which does not ionize to give cyanide ion, here the lone pair of electrons present on nitrogen atom attacks alkyl halide to give alkyl isocyanide. Eg.

4. Substitution by alkoxy group [ Formation of ether] :
Alkyl halide when treated with sodium or potassium alkoxide gives ether. This reaction is known as Williamson’s ether synthesis. Eg.

5. Substitution by amino group [ Formation of amines ] :
When a mixture of haloalkane and alc. NH3 is heated it gives primary amine.

If the haloalkane is present in excess, secondary and tertiary amines are formed. Finally, tertiary amine reacts with haloalkane to give quaternary ammonium salt as the final product.

6. Substitution by nitro group (-NO2) : [Formation of nitroalkane]
Alkyl halide when treated with alcoholic solution of silver nitrite (AgNO2) gives nitroalkane.

7. Substitution by nitrite group (-O-N=O) : [Formation of alkyl nitrite]
Alkyl halide reacts with sodium or potassium nitrite to form alkyl nitrite.

Note :
Q) Haloalkane gives alkyl nitrite with KNO2 while nitroalkane with AgNO2, why?
Nitrite ion is an ambient nucleophile which has two sites i.e. oxygen and nitrogen through which it can attack alkyl halide. KNO2 is an ionic compound which ionizes to give nitrite ion [O-N=O]–, the negative charge of which attacks the alkyl halide to give alkyl nitrite. But silver nitrite is a covalent compound which does not ionize to give nitrite ion, here the lone pair of electrons present on nitrogen atom attacks alkyl halide to give nitroalkane. Eg.
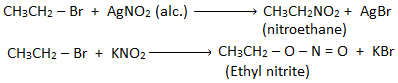
2. Elimination reaction
- When an alkyl halide is heated with alcoholic solution of KOH, then a molecule of hydrogen halide is eliminated from the haloalkane and alkene is formed. Therefore this reaction is also called dehydrohalogenation reaction. Eg.
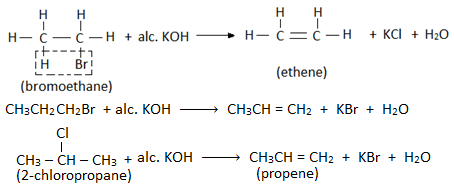
- Elimination reaction involves the removal of halogen atom of haloalkane and a hydrogen atom from the β- carbon (i.e. adjacent carbon). Therefore, this reaction is also known as β- elimination reaction.
- If two or more than two elimination products can be obtained from an alkyl halide then elimination takes place according to Saytzeff’s rule.
Saytzeff’s rule ( or Zaitsev rule) :
When two β- carbon atoms are present, then the elimination of H-atom takes place from the β- carbon atom with fewer number of H- atoms i.e. highly substituted alkene is the major product of dehydrohalogenation reaction. Eg.

3. Reaction of haloaklanes with metals
1. Reaction of haloalkanes with Na (Wurtz reaction) :
When an alkyl halide( haloalkane) is heated with sodium metal in presence of dry ether, a symmetrical alkane containing double number of carbon atoms than in haloalkane is formed. This reaction is called Wurtz reaction.

This is not good method to prepare unsymmetrical alkane because a mixture of two different haloalkanes has to be used which gives a mixture of three different alkanes.
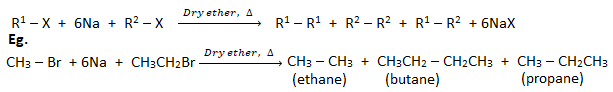
2. Reaction with magnesium :
Alkyl halide when treated with magnesium in the presence of dry ether gives alkyl magnesium halide which is known as Grignard reagent.

4. Reduction reaction of haloalkanes
Haloalkane on reduction gives the corresponding alkane. Reduction of haloalkane can be carried out by using reducing agent like (a) Zn/HCl , (b) Sn/HCl , (c) Na/ethanol , (d) H2/Ni , (e) Lithium aluminium hydride (LiAlH4), (f) Zn-Cu couple , (g) HI/Red phosphorus.
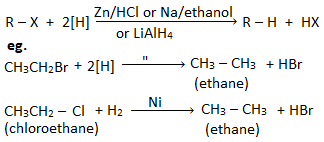
Chloroform (trichloromethane) (CHCl3) :
Preparation of Chloroform:
Chloroform is prepared by heating ethanol or acetone with aqueous bleaching powder paste. Bleaching powder paste acts as oxidizing, chlorinating and hydrolyzing agent.

From ethanol:
Step I : Oxidation :

Step II : Chlorination :

Step III : Hydrolysis :

From acetone (propanone):
Step I : Chlorination :

Step II : Hydrolysis :

Physical properties :
- Chloroform is a colourless sweet smelling liquid.
- It’s freezing point is – 630C and boiling point is 610 C.
- It is heavier than water.
- Chloroform is slightly soluble in water but soluble in ether, alcohol, etc.
- As inhaling of the vapours of chloroform induces unconsciousness therefore it can be used as anaesthetic agent for surgery.
Chemical properties :
1. Reaction with air :
In the presence of sunlight, chloroform is oxidized by air to produce highly poisonous gaseous compound called phosgene (carbonyl chloride).

Thus, chloroform is stored in a dark/coloured bottle to prevent the oxidation of chloroform into phosgene.
A small amount of ethanol is also added into the chloroform at the time of packing because ethanol converts highly poisonous phosgene ( if formed) to a non poisonous diethyl carbonate.

2. Reaction with aq. KOH solution:
When boiling with aqueous KOH solution, chloroform is hydrolysed to form potassium formate which on acidification gives formic acid.

3. Reaction with silver powder:
Chloroform when heated with silver powder gives acetylene(ethyne).

4. Reaction with primary amines (carbylamine reaction):
when chloroform is warmed with a primary amine in the presence of alcoholic KOH, an offensive(unpleasant) smell of carbylamines ( i.e. isocyanide) is obtained. This reaction is known as carbylamine reaction.

Secondary and tertiary amines do not respond to this reaction and therefore, this reaction is used as test reaction for primary amines.
5. Reaction with phenol (Riemer- Tiemann reaction):
When chloroform is heated with phenol and sodium hydroxide followed by hydrolysis, o – hydroxy benzaldehyde ( salicylaldehyde) is formed. This reaction is called Riemer – Tiemann reaction.

6. Reaction with acetone(propanone):
Chloroform reacts with acetone in presence of a base such as KOH to give chloretone.

Chloretone is used as a sleep – inducing (hypnotic) drug.
7. Reaction with HNO3 :
On heating with conc. HNO3, chloroform gives chloropicrin.

Chloropicrin is used as an insecticide and tear gas.
8. Reduction:
Chloroform on reduction with zinc dust and hydrochloric acid (i.e. acidic medium) gives methylene chloride (dichloromethane).

However, when reduced with zinc and water (i.e. neutral medium) gives acetylene (ethene).

Uses of chloroform :
- It is used as an anesthetic. It is now being replaced by other safe anesthetics because chloroform in some cases causes cardiac and respiratory failure.
- It is used as a laboratory reagent for testing primary amines.
- It is used for the preparation of chloropicrin, chloretone, salicylaldehyde, etc.
- It is used in medicines such as a cough syrups.
- It is used as preservative for biological specimens.

Questions and Answers
Questions and their Answers
1. Write the importance and limitations of Wurtz reaction.
Importance : Wurtz reaction is used to prepare the higher members of saturated hydrocarbons ( alkanes) from lower member of alkanes. Eg.

Limitations :
- Methane can not be prepared by this method.
- Tertiary alkyl halides do not undergo this reaction.
- It is not a good method to prepare unsymmetrical alkane because a mixture of two different haloalkanes has to be used which gives a mixture of three different alkanes.

2. How is Grignard reagent prepared? What precautions should be taken for the preparation of Grignard reagents?
Grignard reagent is the alkyl magnesium halide. It is organometallic compound which is represented by the general formula’ RMgX’. Eg.
CH3MgBr ( Methyl magnesium bromide)
CH3CH2MgI ( Ethyl magnesium iodide)
Preparation : Grignard reagent can be prepared by heating haloalkanes or haloarenes with magnesium in the presence of dry ether.
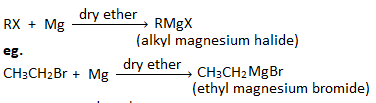
Precautions :
- Grignard reagent is very sensitive to water. When it comes in contact with water, it converts to alkane.

Therefore, during preparation of Grignard reagent there should not be the presence of water molecules i.e. all the reagents should be anhydrous and apparatus oven dried.
- There should not be naked flames nearer.
3. Identify P and Q and write their IUPAC name.

Ans.

Hence,
P = 2-bromopropane
Q = 2,3-dimethylbutane
4. Identify A and B.

Ans. A = propene
B = 2-bromopropane
5. Identify A and B and write their IUPAC name and reaction involved.

Ans.

Hence, A = Ethylmagnesium bromide
B = ethane.
6. Starting from methyl magnesium bromide, how would you prepare methane?
Methane can be prepared by boiling methyl magnesium bromide with water.
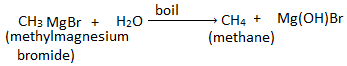
7. Identify A, B, C and D.

Ans.

Hence, A = 2-chloropropane
B = propene
C = ethanal
D = methanal
8. Identify A and B.

Ans. A = ethene and B = methanal
9. Identify A, B, C, D, E and F.

Ans.

10. How will you convert 1-bromopropane to 2-bromopropane and vice-versa.
Ans. 1- bromopropane to 2-bromopropane :
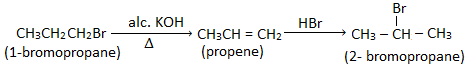
2- bromopropane to 1-bromopropane :

11) Why does chloroform does not give white precipitation with aqueous silver nitrate solution?
→ Chloroform does not give white precipitation with aqueous silver nitrate because C – Cl bond in chloroform is covalent and non – polar and does not ionize in aq. Solution to produce chloride ion (Cl –).
If chloroform is impure, precipitation will occur with aqueous AgNO3 because it may contains HCl as impurities.

12) Write the action of monohydroxy benzene with trichloromethane in presence of alcoholic caustic soda.

13) Identify the products of given reactions.

Answer :


Exercise
1. An organic compound ‘A’ on catalytic reduction gives ‘B’. ‘B’ on chlorination gives ‘C’, ‘C’ on heating with sodium metal in presence of dry ether gives ‘D’. ‘D’ on chlorination gives 2-chlorobutane as major product. Identify A, B, C and D.
2. An organic compound ‘P’ on catalytic reduction gives ‘Q’. ‘Q’ on chlorination gives ‘R’. ‘R’ on heating with sodium metal in presence of dry ether gives ‘S’. ‘S’ on chlorination gives 2-chlorobutane as major product. Give IUPAC names for P, Q, R and S.
3. A primary alkyl halide ‘A’, C4H9Br reacted with hot alcoholic NaOH to give compound ‘B’. Compound ‘B’ reacted with HBr to give ‘C’ which is an isomer of ‘A’. When ‘A’ was reacted with sodium metal it gave a compound ‘D’, C8H18 which was different than compound when n-butyl bromide was reacted with sodium. Give the structural formula and IUPAC name of ‘A’ and write all concerned reactions.
4. A chloro compound ‘A’ on reduction with Zn-Cu couple and alcohol gives the hydrocarbon ‘B’ with five carbon atoms. When ‘A’ is dissolved in ether and treated with sodium 2,2,5,5-tetramethyl hexane is obtained. Write the structure and IUPAC name of ‘A’ and ‘B’.
5. A primary haloalkane ‘P’ when allowed to react with KCN yields a compound ‘Q’,which on acidic hydrolysis gives propanoic acid. Identify ‘P’ and ‘Q’.
6. Compound ‘A’ with the molecular formula C4H9Br is treated with aq. KOH solution. The rate of this reaction depends upon the concentration of the compound ‘A’ only. When another optically active isomer ‘B’ of this compound was treated with aq.KOH solution, the rate of reaction was found to be dependent on concentration of compound and KOH both.
a. Write down the structural formula of both compounds ‘A’ and ‘B’.
b. Out of these two compounds, which one will be converted to the product with inverted configuration?
7. An organic compound (A) having molecular formula C3H7Cl on reaction with alcoholic solution of KCN gives compound B. The compound B on hydrolysis gives compound C. C on reduction with H2 / Ni gives 1-aminobutane. Identify A, B and C.
8. An organic compound ‘A’ reacts with alcoholic KOH to give ‘B’. ‘B’undergoes ozonolysis and gives two compounds ‘C’ and ‘D’ of molecular formula C3H6O. C and D are functional isomers of each other.
a. Write chemical equation for the conversion of A into C and D.
b. Write the structural formula of C and D. why are they called functional isomers?
c. What happens when hydrogen gas in presence of nickel catalyst is passed over ‘X’.
d. What is the application of ozonolysis in the organic reaction mechanism?
e. How can you prove chemically the compound X is unsaturated?
9. A secondary haloalkane X on dehydrohalogenation gives Y. Y on ozonolysis followed by hydrolysis gives ethanal and methanol as major product .
a. Identify X and Y
b. Write all chemical reactions involved
c. What happens when X is heated with sodium metal in presence of dry ether?
d. How would you distinguish X from propane?
10. An organic compound ‘A’ can be used as an anesthetic gives acetylene when heated with silver powder.
a. Write chemical reaction involved in the preparation ‘A’ from ethanol.
b. What precautions should be followed during the storage of ‘A’? Explain with proper reactions.
c. What happens when ‘A’ is heated with aq.KOH solution?
d. How would you prepare chloretone from ‘A’?
11. Differentiate between SN1 and SN2 reaction.
Convert:
- Methane to ethane
- Ethane to ethene
- Methane to ethanoic acid
- Ethanol to ethyne
- Ethane to propanamine
- Chloroethane to propan-2-ol
- 1-bromopropane to 2-bromopropane and vice-versa
Write one example of each:
- Carbylamines reaction
- Riemer-Tiemann reaction
- Dehydrohalogenation reaction
- Wurtz reaction
- SN1 reaction
- SN2 reaction
Account for the following:
- Chloroform is stored in a dark bottle filling upto the brim.
- Small amount of ethanol is added in the bottle of chloroform.
- Chloroform does not give white precipitate with aqueous silver nitrate.
- SN2 reaction gives inverted product.
- Ethanol is soluble in water but ethyl chloride is not soluble.
- Boiling point of n-butyl alcohol is higher than that of tert-butyl alcohol.
- chloroform is not used as an anesthetic these days.
- alkyl halide gives alcohol with aq. KOH while with alcoholic KOH gives alkene.
- Haloalkane reacts with KCN to give alkyl cyanide but with AgCN gives alkyl isocyanide.
References
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Morrison, R.T. , Boyd, R.N., Organic Chemistry, Sixth edition, Prentice-Hall of India Pvt. Ltd., 2008.
- March, j., Advanced Organic Chemistry, Fourth edition, Wiley Eastern Ltd. India, 2005.
- https://en.wikibooks.org/wiki/Organic_Chemistry/Haloalkanes
- https://www.sciencedirect.com/topics/chemistry/haloalkane
- https://www.organic-chemistry.org/namedreactions/wurtz-reaction.shtm
Carboxylic Acids- Aliphatic and Aromatic – Preparation and Properties
Contents [hide]
Carboxylic acids
Organic compounds containing carboxyl group (–COOH) as functional group are called carboxylic acids. Examples:

Classification of Carboxylic acids
On the basis of number of –COOH groups in their molecules carboxylic acids are classified as:
1. Monocarboxylic acids: The carboxylic acids containing one –COOH group in their molecule.

2. Dicarboxylic acids: The carboxylic acids containing two –COOH groups in their molecule.

3. Tricarboxylic acids: The carboxylic acids containing three –COOH groups in their molecule.

Isomerism in carboxylic acids
1. Chain isomerism: eg.

2. Functional isomerism:
Monocarboxylic acids show functional isomerism with ester. Eg.

General methods of preparation of monocarboxylic acids
1. By the oxidation of primary alcohols and aldehydes:
Primary alcohols are easily oxidized first to aldehyde and then to carboxylic acids. Eg.

2. By hydrolysis of alkyl cyanides [i.e. alkane nitriles]:
Complete hydrolysis of alkane nitriles give carboxylic acids. Eg.

3. By hydrolysis of 1,1,1-trihalides:
Carboxylic acids are obtained when 1,1,1-trihalides are hydrolysed in presence of strong alkalies like KOH. The unstable intermediate formed undergoes dehydration to give carboxylic acid. Eg.


4. From Grignard reagent:
When carbon dioxide gas is bubbled into the ethereal solution of Grignard reagent followed by subsequent hydrolysis with dilute acid then carboxylic acid is obtained.

5. From sodium alkoxides and carbonmonoxide:
When sodium alkoxide is heated with CO under pressure it gives sodium salt of carboxylic acid which upon subsequent acidification gives carboxylic acid. Eg.

6. From dicarboxylic acid:
A dicarboxylic acid having two –COOH groups on same carbon atom on heating undergoes decarboxylation giving a monocarboxylic acid. Eg.

Formic acid is prepared in the laboratory by the decarboxylation of oxalic acid with glycerol at 1100C.

7. Preparation of benzoic acid from the oxidation of alkyl benzene:
Benzoic acid can be obtained by oxidation of alkyl benzene with acidic KMnO4 and K2Cr2O7. During oxidation, side chain is oxidized to –COOH group irrespective of the length of the carbon chain. Eg.

Physical properties of carboxylic acids
1. Solubility: The first four carboxylic acids are soluble in water, the next two acids are slightly soluble. Acids having seven or more carbon atoms are insoluble in water. This is due to the fact that lower acids can form H-bond with water but with increased number of carbon atom the polarity of molecules decreases and it cannot form H-bond.
However, all carboxylic acids are soluble in less polar organic solvents such as ether, alcohol, etc.

2. Boiling point:
(Q) The boiling point of methanoic acid is higher than ethanol though they have same molecular mass, explain.
The boiling point of carboxylic acids is much higher than those of alcohols of comparable molecular mass. This is due to the fact that acids form stronger intermolecular H-bond than alcohol as the O-H bond in acids is more polarized due to presence of adjacent electron withdrawing C=O group. It is also due to the fact that carboxylic acid can form a cyclic dimer by forming hydrogen bond between –COOH group.

Acidity of carboxylic acids
Due to presence of polar O-H group, carboxylic acids ionize to give proton and hence behave as acids.

Both carboxylic acid as well as carboxylate anion are stabilized by resonance.
The resonating structures of carboxylic acid are:

The resonating structures of carboxylate anion are:

However, carboxylate anion is more stabilized by resonance as both the resonating structures of carboxylate anion are equivalent. Due to the more stabilized carboxylate ion the equilibrium lies very much in forward direction. Hence, carboxylic acids behave as acids.
Effect of substituents on acidic strength of carboxylic acids:
1. Effect of electron donating (releasing) substituent :
Q) Why is methanoic acid stronger than ethanoic acid?
Positive inductive effect (+I effect) of electron donating(releasing) groups like alkyl groups( CH3 – , CH3CH2 -, etc.) increases the electron density on O – H bond(group). It makes the release of H+ ion difficult. Therefore, the acidic nature decreases with the increase in + I effect. Hence, methanoic acid (formic acid) is stronger acid than ethanoic acid (acetic acid).


2. Effect of electron withdrawing substituent :
Q) Why is chloroacetic acid stronger than acetic acid?
Negative inductive effect (- I effect) of electron withdrawing groups like halogens, – NO2, -CN, etc. decreases the electron density on O – H bond (group). It makes the release of H+ ion easier. Therefore, the acidic nature increases with the increase in – I effect. Hence, chloroacetic acid is stronger acid than acetic acid.


Chemical properties of carboxylic acids
1. Acidic nature:
a. Reaction with metals:
Carboxylic acids react with active metals like Na, K, Ca, Zn, Mg, etc. forming their respective salt and hydrogen gas. Eg.

b. Reaction with alkalies:
Carboxylic acids can neutralize alkalies like NaOH or KOH to form salt and water. Eg.

c. Reaction with carbonates and bicarbonates:
Carboxylic acids decomposes metal carbonates and bicarbonates which produces effervesce due to liberation of CO2 gas. Eg.

4. Reaction with metal oxides:
Carboxylic acids react with basic metal oxides to form salt and water. Eg.

2. Reactions involving cleavage of –OH group:
a. Reaction with alcohols ( Formation of ester) :
Carboxylic acids react with alcohols in the presence of conc. H2SO4 or dry HCl to form esters. This reaction is called esterification reaction. Eg.

b. Reaction with ammonia(Formation of amide) :
Carboxylic acids react with ammonia to form ammonium salt which on heating give amides. Eg.

c. Reaction with PCl5, PCl3 or SOCl2 ( formation of acid chloride) :
Carboxylic acids react with phosphorus pentachloride(PCl5), phosphorus trichloride (PCl3) or thionyl chloride (SOCl2) to form acid chloride. Eg.

d. Formation of acid anhydrides (Dehydration):
Carboxylic acids on heating in the presence of dehydrating agent like P2O5 form acid anhydrides. Eg.

3. Reduction:
If carboxylic acids are reduced with Lithium aluminium hydride (LiAlH4), only the CO group of carboxylic acid is reduced to CH2 to yield alcohols. Eg.

4. Reactions involving alkyl group:
Halogenation : Hell-Volhard Zelinsky [HVZ] reaction:
Carboxylic acids (except formic acid) reacts with chlorine or bromine in presence of red phosphorous to give α-chloro or α-bromo acids. The reaction does not stop at monosubstituted product but continues till all α-hydrogen atoms are replaced.

Abnormal behaviour of formic acid
In formic acid molecule, the carboxylic acid group is attached to hydrogen atom (not to an alkyl group as in case of other higher members). As a result, it possesses dual functional groups i.e. carboxylic group as well as aldehyde group.

Hence, it behaves as an acid and an aldehyde.
1. Action with Tollen’s reagent: When formic acid is boiled with Tollen’s reagent, a silver mirror is deposited.

Acetic acid does not give this test.
2. Action with Fehling’s solution: When formic acid is warmed with Fehling’s solution, a brick red ppt. of cuprous oxide is obtained.

Acetic acid does not give this test.
Reactions of aromatic carboxylic acid (Benzoic acid)
1. Reactions due to carboxyl group:

Reaction due to benzene ring:
-COOH group present in benzoic acid is electron withdrawing group. Thus it deactivates benzene ring by decreasing electron density at ortho- and para- position. Electron density at meta position is comparatively high and hence electrophile attacks the benzene ring at meta position to give meta substituted product.


Cannizzaro’s reaction and Crossed Cannizzaro’s reaction – Definition, Examples and Mechanism
Contents [hide]
Cannizzaro’s reaction
Aldehydes which do not contain α-hydrogen like HCHO, C6H5CHO,etc. undergo self oxidation and reduction on treatment with conc. alkali. In this reaction one molecule is oxidized to carboxylic acid and other molecule is reduced to alcohol. Thus, a mixture of an alcohol and a salt of carboxylic acid is formed by Cannizzaro’s reaction
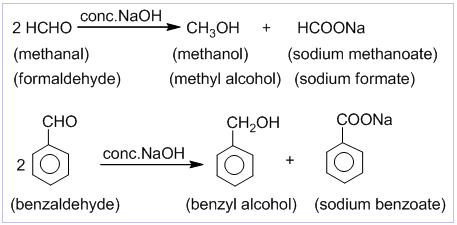
Mechanism of Cannizzaro’s reaction
Taking benzaldehyde as an example, Cannizzaro’s reaction involves following steps:
Step I : In first step, there is nucleophilic addition of the hydroxide ion on the carbonyl group to give anion.
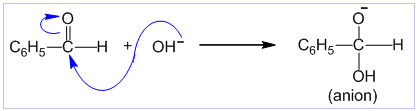
Step II : In this step, the anion transfers a hydride ion to the electron deficient carbonyl carbon of another aldehyde molecule to give carboxylic acid and alkoxide ion.
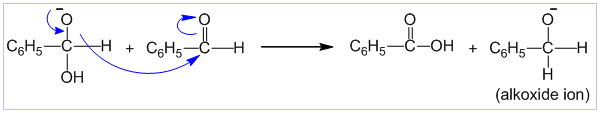
Step III : In this step, there is proton exchange between the carboxylic acid and the alkoxide ion to give salt of carboxylic acid and alcohol.

Crossed Cannizzaro’s reaction
When a mixture of formaldehyde and other aldehyde which has no α-hydrogen atom is treated with conc. alkali, formaldehyde is oxidized to carboxylic acid and another aldehyde is reduced to alcohol. This reaction is crossed cannizzaro’s reaction.
For example, benzyl alcohol and sodium formate is formed when a mixture of benzaldehyde and formaldehyde is treated with conc. NaOH.
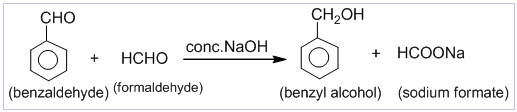
Mechanism of crossed cannizzaro’s reaction
Taking a mixture of benzaldehyde and formaldehyde as an example, crossed cannizzaro’s reaction involves following steps:
Step-I : In first step, there is nucleophilic addition of the hydroxide ion on the carbonyl group of formaldehyde to give anion.

Step II : In this step, the anion transfers a hydride ion to the electron deficient carbonyl carbon of benzaldehyde molecule to give carboxylic acid and alkoxide ion.

Step III : In this step, there is proton exchange between the carboxylic acid and the alkoxide ion to give salt of carboxylic acid (sod. formate) and alcohol( benzyl alcohol).

Advantage of crossed- cannizzaro’s reaction:
In this reaction an aldehyde is treated with formaldehyde. Here, formaldehyde is oxidized to sodium formate and required alcohol is obtained from the reduction of the other aldehyde. Since both the aldehydes used are completely converted into the required products, there is no wastage of the valuable chemicals.
Why does methyl alcohol is not formed instead of sodium formate in crossed cannizzaro’s reaction?
The initial nucleophilic addition of hydroxide ion is faster on formaldehyde than on other aldehyde as there is no electron donating groups on formaldehyde. Hence, formic acid (sodium formate) is formed but not methyl alcohol.
References
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Morrison, R.T. , Boyd, R.N., Organic Chemistry, Sixth edition, Prentice-Hall of India Pvt. Ltd., 2008.
- March, j., Advanced Organic Chemistry, Fourth edition, Wiley Eastern Ltd. India, 2005.
- https://www.toppr.com/ask/content/story/amp/crossed-cannizaro-reaction-6282/
- https://chemicalnote.com/aldol-condensation-and-crossed-aldol-condensation-reaction-definition-examples-mechanism-and-uses/.
Reaction mechanism – Methods of determining reaction mechanism.
Contents [hide]
Reaction mechanism
A reaction mechanism is the actual process by which a reaction takes place. It explains which bonds are broken, in what order, how many steps are involved, the relative rate of each step etc.
- The reaction mechanism describes the sequence of elementary reactions that must occur to go from reactants to products.
- Reaction intermediates are formed in one step and then consumed in a later step of the reaction mechanism.
Types of mechanism
Depending on how the bonds break, organic mechanisms can be divided into three basic types.
a. Homolytic or free radical mechanism :
If a bond breaks in such a way that each fragment gets one electron , free radicals are formed and such reactions are said to take place by hemolytic or free radical mechanism.

b. Heterolytic mechanism :
If a bond breaks in such a way that both bonding electrons remain with one fragment, ions are formed and such reactions are said to take place by heterolytic mechanism.

c. Pericyclic mechanism :
This mechanism involves the cyclic movements of the electrons in the bond breaking and bond making. There are no intermediates, ions or free radicals. Reactions with this type of mechanism are called pericyclic mechanism.

Thermodynamic and kinetic requirements for a reaction
Thermodynamic requirements for a chemical reaction :
For a reaction to take place spontaneously, the free energy of the products must be lower than the free energy of the reactants. i.e. ∆G must be negative.
We know that
∆G = ∆H – T∆S
Where,
∆G = Change in free energy
∆H = Change in enthalpy
∆S = Change in entropy
T = temperature
To be ∆G negative, there should be decrease in enthalpy and increase in entropy of the system.
For many reactions entropy effects are small and it is the enthalpy that mainly determines whether the reaction can take place spontaneously. However, in certain types of reaction entropy is important and can dominate enthalpy. Some of the examples are:
- Generally, gases have higher entropy than liquids and solids because the molecules of gas have much more randomness. Liquids have higher entropy than that of solids but lower than that of gases. Hence, any reactions in which the reactants are solids and the products are liquids and gases are thermodynamically favored.
- When the number of products formed are more than that of reactants, degree of freedom increase and hence entropy also increases making the reaction thermodynamically favored. On the other hand, reactions in which the number of product molecules is less than the number of reactant molecules, entropy decreases and in such cases there must be significantly decrease in enthalpy to be ∆G negative.
- An open chain molecule has more entropy than a similar cyclic molecule because there are more conformations. Hence, ring opening reactions are thermodynamically favorable.
Kinetic requirements for a chemical reaction :
A negative ΔG is a necessary but not a sufficient condition for a reaction to occur spontaneously. For example, the reaction between H2 and O2 to give H2O has a large negative ΔG, but mixtures of H2 and O2 can be kept at room temperature for many centuries without reacting to any significant extent.
So, for a reaction to occur, the chemical reactions require some activation energy. Activation energy is defined as the minimum amount of extra energy required by a reacting molecule to get converted into product. It can also be described as the minimum amount of energy needed to activate or energize molecules or atoms so that they can undergo a chemical reaction or transformation.

In the above energy profile diagram, ∆Gf is the free energy of activation for forward reaction and ∆Gr is the free energy of activation for backward reaction.
Kinetically vs Thermodynamically controlled reaction
Q) Give labeled energy profile diagram illustrating kinetic versus thermodynamic control of the product.
Let us consider a chemical reaction in which a reactant ‘A’ gives two different products ‘B’ and ‘C’ by different mechanism.

In this figure, ‘C’ is thermodynamically more stable ( due to lower energy ) but ‘B’ is formed faster ( due to lower energy of activation).
If the reaction is irreversible, energy of activation is lower for the formation of B, so B is formed faster. So, the product ‘ B’ is said to be kinetically controlled.
However, if the reaction is permitted to approach equilibrium (i.e. if the reaction is reversible), the more stable product C predominates. Under these conditions, the product B that is first formed reverts to A. So, the product ‘C’ is said to be thermodynamically controlled.
Example of kinetic and thermodynamic control of the reaction :
Q) Explain the term kinetic and thermodynamic control of the reaction with suitable examples.
Q) Give the mechanism of kinetically controlled and thermodynamically controlled addition of HBr to 1,3-butadiene.
The kinetic and thermodynamic control of the reaction can be understood by taking an example of addition of HBr to 1,3-butadiene.
Addition of HBr to 1,3-butadiene gives two types of products- 1,2-addition product and 1,4-addition product.

Mechanism :
From the above data, it can be concluded that at – 800C, 1,2-product is formed faster than 1,4-product due to less energy of activation for 1,2-product. 1,2- addition product is formed as a major product. Thus 1,2-addition product is kinetically controlled product.
But when the temperature is raised the equilibrium is attended and the reaction becomes reversible. At this condition, the 1,4-addition product is formed as a major product because it is more stable (less energy) than 1,2-addition product. The 1,2-product is formed faster but it ionizes and converts into more stable 1,4-addition product. Thus 1,4- addition product is thermodynamically controlled.
Hammond postulate
Q) State Hammond postulate. Explain with a suitable example the application of Hammond postulate in determining the shape and geometry of transition state. How would you differentiate intermediate from transition state ?
Q) Give the statement of Hammond postulate. Why is it most useful ?
Transition states have zero life time, so it can not be isolated and studied directly and hence the information about their shape and geometries must be obtained from inference. Hammond postulate states that ‘ for any single reaction step, the geometry of the transition state for that step resembles to the compound (reactants or products) to which it is closer in free energy’.
Thus, for the endothermic reaction the transition state resembles the products more than the reactants because transition state is closer to product in energy profile diagram (i.e. ∆G2<∆G1).
For the exothermic reaction the transition state resembles the reactants more than the products because transition state is closer to reactant in energy profile diagram (i.e. ∆G1<∆G2).

Reactant→T.S.1→Intermediate→T.S.2→Product

The T.S.1 lies much closer in free energy to the intermediate than to the reactants and hence we can predict that the geometry of T.S.1 resembles that of intermediate more than that of reactants. Similarly, T.S.2 also has a free energy much closer to that of the intermediate than to the products. Therefore, both transition states resemble the intermediate more than the reactants and products.
Difference between transition states and intermediates :
The transition state refers to an imaginary molecule having zero life time and cannot be isolated. In this state the system possesses maximum energy and is most unstable. It is impossible to observe them directly and information about their geometries must be obtained from inference.
On the other hand, the relatively stable products formed from the reactants during reaction that further reacts to give the final product is called intermediate. The energy of intermediate is less than that of transition state and can be isolated and studied. Their geometries can be obtained by using many techniques such as IR, NMR spectroscopy, etc. We often use the knowledge of intermediates to determine the shape and geometry of transition states.
Q) State Hammond postulate. What information does the postulate provides about the structure of T.S. in the following energy profile diagram ?

Microscopic reversibility
Under the same reaction condition, if the reaction is reversible, the forward and reverse reactions must proceed by same mechanism. This is called the principle of microscopic reversibility. For example, if in a reaction A → B there is an intermediate ‘C’, then ‘C’ must also be an intermediate in the reaction B → A.

This is a very useful principle to predict the mechanism of reversible reaction.
For example, when alcohol is treated with H2O and H2SO4, alkene is obtained via carbocation intermediate. When same alkene is treated with H2O and H2SO4 , alcohol is obtained via same carbocation intermediate.

Methods of determining reaction mechanism
There are a number of commonly used methods for determining reaction mechanism. In most cases, one method is not sufficient. Reaction intermediates are the important class of chemical species, which are quite helpful in understanding the mechanism of a chemical reaction.
1. Isolation of intermediate :
Isolation of intermediates gives the valuable information to identify the exact mechanism. It is sometimes possible to isolate an intermediate from a reaction mixture by stopping the reaction after a short time or by the use of very mild conditions. For example, Hoffmann’s bromamide reaction:

During this reaction following intermediates were formed and isolated:

In order to explain the formation of these intermediates, following mechanism was proposed:
2. Detection of intermediate :
In many cases, intermediate cannot be isolated but can be detected. It can be detected by IR, NMR or other spectroscopic technique. The detection of aman spectra of NO2+ (nitronium ion) indicates it is an intermediate in nitration of benzene. Hence, nitration of benzene is electrophilic substitution reaction and the following mechanism for the nitration of benzene was proposed:

3. Stereochemical evidence :
If the products of a reaction are capable of existing in more than one stereoisomeric forms, the form which is obtained may give information about the mechanism. For example, in SN2 reaction the product product obtained is always inverted. This is only possible if the attack of nucleophile and removal of leaving group takes place simultaneously. In such cases, nucleophile attacks from backside of leaving group to give inverted product.

4. Identification of products :
Identification of the products of a reaction also helps to define the reaction mechanism.
For example, elimination reaction of 2-bromobutane.

From the identification of but-2-ene as major product and bu-1-ene as minor product, following mechanism can be proposed:

5. Isotope labeling ( Tracer technique) :
Q) What is the scope of isotope labeling (tracer technique) in the determination of reaction mechanism? Explain with suitable example.
When one isotope of the bonded atom is replaced by its heavier isotope, the rate of bond breaking becomes slower. So by comparing the rate of original bond breaking with that of the heavier isotope substituted bond, we can determine whether a particular bond breaks in the rate determining step or not. The effect of heavier isotope on the rate of the bond breaking is called isotope effect.
Much important information can be obtained by using molecules that have isotopically labeled and tracing the path of the reaction in that way.
For example, ester undergoes hydrolysis to form a mixture of carboxylic acid and alcohol.

The products may have been formed by acyl-oxygen bond fission (a) or by alkyl-oxygen bond fission (b). If the hydrolysis of ester is carried out using H2O18 (i.e. oxygen having mass 18), the following products would be expected:

In this reaction, the appearance of O18 in carboxylic acid has been confirmed by mass spectroscopy. So the fission must have occurred at acyl-oxygen bond in the hydrolysis of ester.
Q) Show that hydrolysis of ethyl acetate occurs acyl-oxygen bond fision but not ethyl-oxygen bond fission with the help of tracer technique.
6. Trapping of intermediate :
In some cases, the suspected intermediate is known to be one that reacts in a given way with a certain compound. The intermediate can then be trapped by running the reaction in the presence of that compound.
For example, benzyne reacts with dienes in Diels- Alder reaction. In any reaction where a benzyne is suspected intermediate, the addition of a diene and the detection of Diels-Alder addition product indicates that the benzyne was present.

Q) The action of p-chlorotoluene with sodamide in presence of liquid ammonia yields a mixture of para and meta toluidene. Give the mechanism of the reaction.
Q) Using the method of trapping intermediate, predict the suitable mechanism for the following reaction:

Answer :
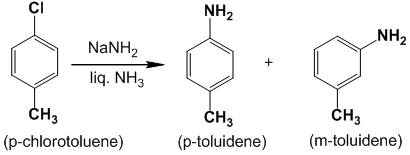
The mechanism of this reaction is believed to proceed as follows by trapping benzyne intermediate.
Step – I : The first step involves the loss of H+ and Cl- from p-chlorotoluene to form a benzyne intermediate.

Step – II : The second step involves the attack of the benzyne intermediate by NH2– followed by protonation.
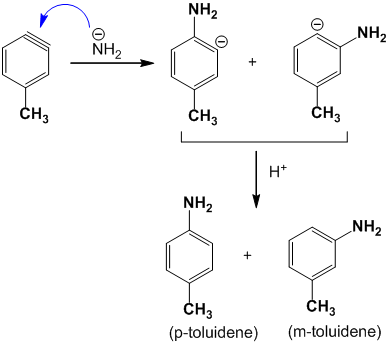
Baldwin’s rule of ring closure
J.E Waldwin has proposed a set of rules for ring closure reactions of 3 to 7 membered rings. The process could be ‘endo’ and ‘exo’. ‘Exo’ means the bond broken during the ring closure is outside while the ‘endo’ means the bond broken during the ring closure is inside.

The hybridization of carbon atom (C) undergoing the ring closure reaction is of three types :
Tetrahedra (Tet) – sp3 (i.e. a single bond centre)
Triginal (Trig) – sp2 (i.e. a double bond centre)
Diagonal (Dig) – sp (i.e. a triple bond centre)
The following are Baldwin’s rule for closing rings of 3 to 7 members:
Rule 1 : Tetrahedral system
- 3 to 7 – Exo – Tet are all favored
- 5 to 6 – Endo – Tet are disfavored
Rule 2 : Trigonal system
- 3 to 7 – Exo – Trig are favored
- 3 to 5 – Endo – Trig are disfavored
- 6 to 7 – Endo – Trig are favored
Rule 3 : Diagonal system
- 3 to 4 – Dig are disfavored
- 5 to 7 – Dig are favored
- 3 to 7 – Dig are favored
Disfavored does not mean it cannot occur but only it is more difficult than the favored cases. A reaction that is disfavored (slow) does not have a rate that is able to compete effectively with an alternative reaction that is favored (fast).
Example: 5 – Exo – Trig
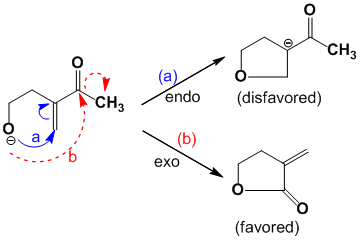
References
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Morrison, R.T. , Boyd, R.N., Organic Chemistry, Sixth edition, Prentice-Hall of India Pvt. Ltd., 2008.
- March, j., Advanced Organic Chemistry, Fourth edition, Wiley Eastern Ltd. India, 2005.
- http://epgp.inflibnet.ac.in/epgpdata/uploads/epgp_content/chemistry/05.organic_chemistry-ii/03.thermodynamic_and_kinetic_requirements_of_a_reaction/et/5547_et_et.pdf
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Equilibria/Chemical_Equilibria/Principles_of_Chemical_Equilibria/Kinetically_vs_Thermodynamically_Stable
- https://www.mt.com/my/en/home/applications/L1_AutoChem_Applications/L2_ReactionAnalysis/reaction-mechanisms.html
- https://www.intechopen.com/books/chemometrics-in-practical-applications/analysis-of-chemical-processes-determination-of-the-reaction-mechanism-and-fitting-of-equilibrium-an
Phenols – Nomenclature, Preparation and Properties
Phenols are hydroxyl derivatives of benzene in which –OH group is directly attached to benzene ring. Eg.
If –OH group is attached to the side chain of an aromatic ring then it isn’t considered as phenol. This type of compounds are known as aromatic alcohol. Eg.

Contents [hide]
Nomenclature of phenols

General methods of preparation of phenol
1. From diazonium salt (Laboratory method):
Phenol is prepared in the laboratory by warming aq. solution of benzene diazonium chloride.

2. From chlorobenzene (Dow’s process):
Chlorobenzene when heated with aq. solution of NaOH (or Na2CO3) at about 3500C under 300 atmospheric pressure gives sodium phenoxide which upon acidification gives phenol.

3. From sodium salicylate:
Sodium salicylate on distillation with sodalime undergoes decarboxylation to form sod. phenoxide which on acidification gives phenol.

4. From Grignard reagent:
When oxygen is bubbled through an ethereal solution of phenyl magnesium bromide, it forms an addition product which upon acidic hydrolysis gives phenol.

5. From methoxy benzene(anisole):
Methoxy benzene when heated with HI gives phenol.

Q) How will you convert phenol into anisole (methoxy benzene) and vice-versa.

Physical properties of phenol
1. Solubility of phenols:
Phenols are slightly (sparingly) soluble in water though it can form intermolecular H-bond with water molecules. Limited solubility is mainly due to hydrophobic nature of benzene ring.

2. Melting and boiling point of phenols:
The melting and boiling points of phenols are higher than the arenes and holoarenes of comparable molecular masses. This is due to the presence of intermolecular H-bond between phenol molecules.

Chemical properties of phenol
In general, chemical reactions of phenols are of two types.
1. Reactions due to phenolic(-OH) group.
2. Reactions due to benzene ring.
Reactions due to phenolic(-OH) group
1. Acidic nature of phenols:
Phenols turn blue litmus red i.e. phenols behave as weak acids and react with metals and alkalies as follows:

Phenols do not react with Na2CO3 or NaHCO3 as they are weaker acids than carboxylic acids.
Q) Phenols are stronger acids than alcohols, why?
Phenols are more acidic alcohols. This can be explained by considering the relative stabilities of phenols and phenoxide ions as compared to alcohols and alkoxide ions respectively.
Phenols as well as phenoxide ions both are resonance stabilized and their resonating structures are given below:

The resonating structures of phenol involves charge separation, whereas in case of phenoxide ion there is no charge separation. From this it is clear that phenoxide ion is more stabilized by resonance than phenol. Therefore, in the dissociation of phenol, equilibrium shifts to the forward direction and forms relatively high concentration of H+ ions and behave as strong acids compared to alcohols.

On the other hand, in the case of alcohols neither alcohols nor alkoxide ions are stabilized by resonance, and hence they behave as weaker acids than phenol.

Hence,

2. Reaction with zinc dust:
When phenol is heated with zinc dust, benzene is formed.

3. Reaction with ammonia: In the presence of anhydrous ZnCl2, phenol reacts with ammonia at high temperature to give aniline.

4.Reaction with acid chlorides or acid anhydrides: (Acylation)
Phenols react with acid chloride in presence of pyridine or with acid anhydrides in the presence of mineral acids like H2SO4 or base pyridine to form esters.

5. Reaction with benzoyl chloride: (Benzoylation)
Phenols react with benzoyl chloride in the presence of aqueous NaOH to form phenyl benzoate.

6. Reaction with phosphorus pentachloride:
Phenols react with PCl5 to produce chlorobenzene.

Reactions due to benzene ring
Electrophilic substitution reaction of phenol:
The resonating structures of phenol are:

It is clear from the above resonating structures that ortho and para positions are relatively rich in electron density and hence incoming electrophile attacks at these positions. Thus, -OH group is an ortho/para directing group towards electrophilic substitution reaction.
1. Halogenation: Phenols react with halogens to form poly halogen substituted compounds. For example, phenol gives white ppt. of 2,4,6-tribromophenol when treated with bromine water.

2. Nitration: Phenol when nitrated with concentrated nitric acid and conc. H2SO4 gives 2,4,6-trinitrophenol (picric acid).

3. Sulphonation: Sulphonation of phenol takes place mainly at ortho-position at low temperature and at para-position at high temperature.

4. Friedel-Craft’s alkylation: Phenols react with alkyl halides in the presence of anhy.AlCl3 to form mainly para-product with a small amount of the ortho-product.

Special reactions of phenol
1. Kolbe’s reaction (Carboxylation reaction):
Sodium phenoxide when heated with CO2 at1350C under a pressure of 4-7 atm, sodium salicylate is obtained which when acidified gives salicylic acid.

Salicylic acid is the starting material for the manufacture of 2-acetoxybenzoic acid (aspirin), the well known analgesic. Aspirin is obtained from salicylic acid by reacting it with acetic anhydride.

2. Reimer-Tiemann reaction: When phenol is refluxed with chloroform and aq. NaOH at 600C, a mixture of o-hydroxybenzaldehyde and p-hydroxybenzaldehyde is obtained. This reaction is called Reimer-Tiemann reaction. 
3. Reaction with phthalic anhydride:
Phenol on condensation with phthalic anhydride in the presence of conc. H2SO4 gives phenolphthalein.

4. Coupling reaction:
Phenols react with benzene diazonium chloride in slightly alkaline medium and at low temperature to form coloured compounds called azo dyes. This reaction is called coupling reaction.

Note: Arene diazonium salts react with highly reactive aromatic compounds like phenol, aniline, etc. to form highly colored azo-compound, (Ar-N=N-Ar). This reaction is known as coupling reaction.
5. Catalytic hydrogenation: Phenol gets hydrogenated in the presence of nickel catalyst at 1500C to give cyclohexanol.

6. Oxidation: On exposure to air, phenol gets slowly oxidized and turns pink in colour.

7. Reaction with ferric chloride: Phenols react with ferric chloride solution to form water soluble coloured complexes.

8. Condensation(polymerization) of phenol with formaldehyde (methanal):
Phenol condenses with formaldehyde in the presence of an acid or basic catalyst to form a polymer called Bakelite.
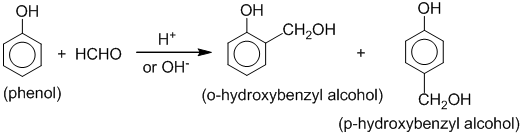
Formation of linear polymer:

Formation of cross-linked polymers:

References
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Morrison, R.T. , Boyd, R.N., Organic Chemistry, Sixth edition, Prentice-Hall of India Pvt. Ltd., 2008.
- Sthapit, M.K., Pradhananga, R.R., Foundations of Chemistry, Vol 1 and 2, Fourth edition, Taleju Prakashan, 2005.
- https://www.vedantu.com/chemistry/preparation-of-phenol#:~:text=In%20this%20method%2C%20benzene%20sulfonic,aqueous%20acid%20to%20make%20phenol.
- https://www.cliffsnotes.com/study-guides/chemistry/organic-chemistry-ii/phenols-and-aryl-halides/synthesis-of-phenols
Contents [hide]
What are Drugs (Medicines) ?
Drugs or medicines are the chemical substances which are used in the prevention, diagnosis and treatment of diseases.
Classification of some common drugs

Antipyretics
- Antipyretics are the drugs which are used to reduce fever i.e. reducing the body temperature to the normal.
- Examples : aspirin, paracetamol, etc.

- Paracetamol is preferred over aspirin, since aspirin gets hydrolysed to salicylate in stomach that may causes bleeding and gastritis.
Analgesics
- The drugs which are used to relieve or decrease pain (without the loss of consciousness) are called analgesics.
- These are also called as pain killers or pain relievers.
- Examples : aspirin, novalgin, analgin, etc.

Antipyretics and analgesics
- Some drugs can be used to reduce fever as well as to relieve pain, such drugs are called antipyretic and analgecics.
- Aspirin is a common example of antipyretic and analgesic drug.
Antibiotics
- Antibiotics are chemical substances produced by various micro-organism (such as bacteria, fungi and molds) and are capable of destroying or suppressing the growth of other microorganism.
- Examples : penicillin, chloramphenicol, etc.
- Antibiotics which are effective against several different types of harmful micro – organism are called broad spectrum antibiotics. eg. chloramphenicol, tetracycline, etc.

- Chloramphenicol is used to cure typhoid, pneumonia, meningitis,etc.
Antiseptics
- Antiseptics are the chemical substances which prevent the growth of micro-organism or kill them ( in wounds, cuts, etc.) but are not harmful to the human beings.
- It is directly applied into the infected parts i.e. they are usually of external use.
- Examples : Dettol, savlon, 0.1% phenol, boric acid, etc.

Disinfectants : Chemical substances which are applied to inanimate objects like instruments, utensils, clothes, etc. Most of them are harmful to the living tissues and thus cannot be used on the skin. Examples : 1% phenol, chlorine, H2O2, etc.
Sulpha (sulfa) drugs
- Sulpha drugs are group of synthetic antibiotics derived from sulphanilamide.
- Sulpha drugs were the first chemical substances systematically used to trat and prevent bacterial infections in humans.
- Their use has diminished because of the availability of antibiotics that are more effective and safer.
- Sulpha drugs are still used, but largely for treating urinary tract infections, dysentery and preventing infection of burns.

Tranquilizers
- Tranquilizers are the chemical substances used to cure mental diseases.
- In short, a tranquilizer restores the peace of mind.
- These are also called psycho – therapeutic drugs.
- Examples : Barbituric acid, Diazepam, alprazolam, clonazepam, etc.
Anti- allergic ( Antihistamines)
- Antihistamines are the drugs used to treat allergies.
- They work by preventing the effects of a substance called histamine, which is produced by the body. Histamine can cause itching, sneezing, runny nose, and watery eyes.
- Examples : fexofenadine, citirizine, etc.
Bronchodilators
- Bronchodilators are the drugs that make breathing easier by relaxing the muscles in the lungs and widening the airways (bronchi).
- They are often used to treat long-term conditions where the airways may become narrow and inflamed, such as- asthma.
- Examples : salbutamol, salmeterol, formoterol, etc.
Antacids
- Antacids are the chemical substances which can reduce or neutralize the acidity in stomach and raise the pH to appropriate level.
- Antacids contain weakly basic chemicals such as magnesium hydroxide i.e. Mg(OH)2, Aluminium hydroxide i.e. Al(Cl)3, magnesium carbonate i.e. MgCO3, etc.
Objective Questions
1. Drugs which helps to reduce anxiety and brings about calmness is
a. Analgesic c. tranquilizer
b. Antibiotic d. Antacids
2. Which of the following is an analgesic drug ?
a. Penicillin c. Chloramphenicol
b. Novalgin d. thymol
3. Which of the following is an antibiotic drug?
a. Aspirin c. Novalgin
b. Valium d. Chloramphenicol
4. Aspirin is
a. Analgesic c. Antibiotic
b. Antipyretic d. Both a and b.
5. 1 % solution of phenol is
a. Antipyretic c. Anti histamine
b. Disinfectant d. Analgesic
6. The use of chemicals for the treatment of diseases is called as
a. Chemotherapy c. Physiotherapy
b. Homeotherapy d. Physiotherapy
7. Tranquilizers are substances used for the treatment of
a. Mental diseases c. Malaria
b. Physical disease d. Cancer
8. Acetyl salicylic acid acts as
a. Antacid c. Antipyretic
b. Antibiotic d. Antiseptic
9. Dettol consists of
a. Xylenol + terpineol
b. Chloroxylenol + terpineol
c. Cresol + ethanol
d. None of the above
10. A broad spectrum antibiotic is
a. Paracetamol c. Penicillin
b. Aspirin d. Chloramphenicol
11. Salbutamol is
a. Antihistamine c. Tranquilizer
b. Bronchodilator d. Antacid
12. Antacids contain
a. Strong acids c. Weak acids
b. Strong bases d. Weak bases
Answer :
1 – c 2- b 3- d 4- d
5- b 6 – a 7- a 8- c
9- b 10- d 11-b 12 – d
References
- Sthapit, M.K., Pradhananga, R.R., Foundations of Chemistry, Vol 1 and 2, Fourth edition, Taleju Prakashan, 2005.
- https://www.drugabuse.gov/drug-topics/commonly-used-drugs-charts
- https://www.fda.gov/drugs
- https://www.nhs.uk/conditions/bronchodilators/
- https://www.webmd.com/allergies/antihistamines-for-allergies
- https://www.webmd.com/allergies/antihistamines-for-allergies
- https://www.addictionsandrecovery.org/benzodiazepine.htm
Aromatic Compounds – Structure, Preparation, Properties and Uses of Benzene.
Contents [hide]
What are aromatic compounds ?
Benzene and those cyclic compounds that chemically behave as benzene are called aromatic compounds. Eg.

Aromatic compounds are also known as arenes (i.e. aromatic alkenes).
{ the term aromatic was derived from the Greek word ‘aroma’ meaning sweet smelling}Structure of benzene :
Kekule’s structure of benzene
Kekule, a German scientist proposed the structure of benzene for the first time. According to Kekule, all the 6 carbon atoms of benzene molecule are joint to each other by alternate single and double bond forming a hexagonal ring and a hydrogen atom is bonded to each carbon atom.

Resonance structure of benzene
The double bonds may be localized in any position and therefore following resonating structures are possible :

According to these structures, there should be three single bonds (bond length 154 pm) and three double bonds (bond length 134 pm) between carbon atoms in the benzene molecule. But actually it has been found by X- ray diffraction studies that all the carbon-carbon bonds in benzene are equivalent and have bond length 139 pm , which is intermediate between C – C (154 pm) and C = C (134 pm). Thus, the actual structure of benzene is different from both ‘A’ and ‘B’ and is a resonance hybrid of these two resonating forms.
{ Note: pm = picometre, 1pm = 10 -12 m}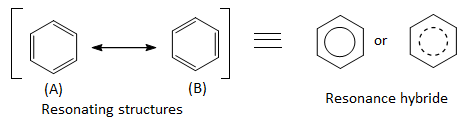
Molecular orbital structure of benzene
Each carbon atom in benzene has to join to three other atoms( one H and two C) and doesn’t have enough unpaired electrons to form the required number of bonds, so it needs to promote one of the 2s2 electron into empty 2pz orbital. This is called excited state configuration.

Because each carbon is only joining to three other atoms, carbon atom is sp2 hybridized using one 2s electron and two 2p electrons leaving one 2p electron unchanged.
Two of the sp2 hybrid orbitals of each of the six carbon atoms overlap with each other to form a hexagonal ring, while rest of the hybrid orbital overlaps with 1s orbital of a hydrogen atom. The unhybridized 2pz orbital of each carbon atom undergoes sidewise overlapping below and above the ring to form π bonds.
Therefore, a benzene molecule consists of twelve σ bonds and three π bonds. It shows the planar type of structure with bond angle 1200 between two carbon atoms.
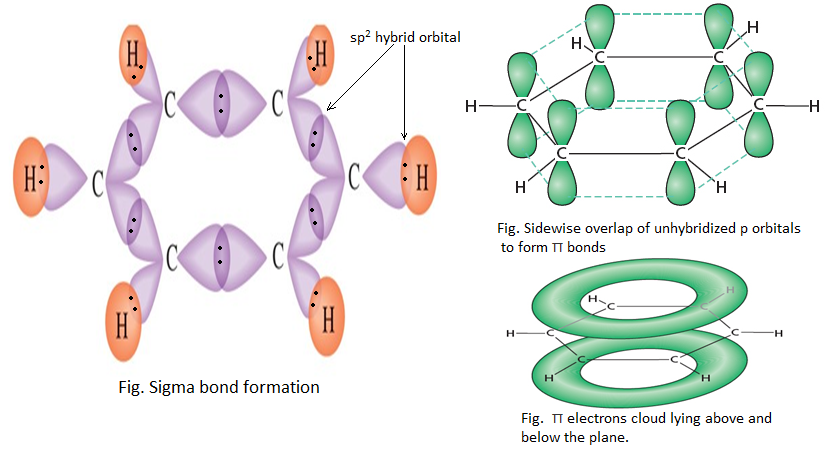
Nomenclature of aromatic compounds
1. Naming of mono-substituted benzene
Monosubstituted benzenes are usually named by prefixing the name of the substituent before the word ‘ benzene’. Eg.

However, some of the monosubstituted benzenes are known by their special names.


2. Naming of disubstituted benzene
A disubstituted benzene shows isomerism ( i.e. position isomerism). The second substituent can enter into any one of the five positions of the ring. Since positions (2 and 6) and (3 and 5) are equivalent positions, there can be three isomers of the disubstituted benzenes. Eg.


- 2 and 6 positions are also indicated by prefix ortho (o-)
- 3 and 5 positions are also indicated by prefix meta (m-)
- Position ‘4’ is also indicated by prefix para (p-). Eg.


If two different substituents are present then ortho, meta and para is followed by the substituent in an alphabetical order. Eg.

If one of the substituent present in the disubstituted benzene gives the special name then disubstituted benzene is named as derivatives of that special molecule. Eg.


If both the substituent present in disubstituted benzene give the special name, then the substituent with higher priority is numbered as 1.
The priority order of functional groups :
-COOH > -SO3H > – COO- > -COX > -CONH2 > -CN > -CHO > -CO- > -OH > -NH2



3. Naming of tri or polysubstituted benzene


Note : In some cases benzene ring is considered as substituent. Eg.

Benzyl, Benzal and Benzo groups

Also see the Nomenclature of Aliphatic compounds …
Necessary conditions for any compound to show aromaticity
The necessary conditions for any compound to show aromaticity are as follows :
- The compound must be cyclic and planar and allow cyclic overlap of p-orbitals.
Note : Usually ring compounds containing upto 7 carbon atoms are planar.
- There must be complete delocalization of π – electrons.
- The compounds must contains π – electrons according to Huckel’s rule, i.e. (4n+2) π – electrons, where n = 0, 1, 2,3, 4, etc.
Huckel’s rule
Huckel’s rule states that a cyclic, planar and conjugated molecule is aromatic if it contains 4n+2 delocalized π electrons, where n = 0, 1, 2, 3, 4, etc.
Examples :
i. Benzene :

i.e. 4n+2 = 6
4n= 4
n = 1(which is an integer)
Therefore, benzene is an aromatic compound. It will show aromaticity.
ii. Cyclooctatetraene :
 It is cyclic, planar and has a cyclic overlap of p-orbitals. There are 4 double bonds and 8 π – electrons which isn’t consistant with Huckel’s rule.
It is cyclic, planar and has a cyclic overlap of p-orbitals. There are 4 double bonds and 8 π – electrons which isn’t consistant with Huckel’s rule.
i.e. 4n+2 = 8
n = 3/2 (which is in fraction)
So, cyclooctatetraene isn’t an aromatic compound. It won’t show aromaticity.
iii. Cyclopentadiene :
 It is cyclic and one of the carbon atom is sp3 hybridized. Hence, the complete overlapping of p- orbitals isn’t possible. There are 4 π – electrons which isn’t consistant with Huckel’s rule.
It is cyclic and one of the carbon atom is sp3 hybridized. Hence, the complete overlapping of p- orbitals isn’t possible. There are 4 π – electrons which isn’t consistant with Huckel’s rule.
i.e. 4n+2 = 4
n = 1/2 (which is in fraction)
So, cyclopentadiene isn’t an aromatic compound. It won’t show aromaticity.
Preparation of benzene
1. From ethyne ( manufacture of benzene) :
When ethyne gas is passed through a red hot iron or copper tube, the three molecules of ethyne (acetylene) polymerize to give benzene.


2. By decarboxylation of sodium salt of benzoic acid ( Laboratory method):
Benzene can be prepared by heating sodium salt of benzoic acid ( i.e. sodium benzoate) with sodalime. This reaction is called decarboxylation reaction.

Note :

3. By reducing phenol with zinc :
When vapours of phenol are passed over heated zinc dust, benzene is produced.
4. By the reduction of aryl halides :
Benzene can be prepared by the reduction of haloarene with nickel – aluminium alloy in the presence of alkali.

Chemical properties of benzene
Electrophilic substitution reactions of benzene
The most important/common reaction of benzene is electrophilic substitution reaction. In this reaction, an electrophile attacks the benzene and substitutes one of the hydrogen atoms of benzene ring. Eg.
1. Halogenation :
Benzene reacts with bromine in presence of ferric bromide as catalyst to give bromobenzene.

Similarly, chlorine reacts with benzene in presence of ferric chloride or AlCl3 as catalyst to give chlorobenzene.

2. Nitration : When benzene is heated with conc. HNO3 in the presence of conc. H2SO4 at about 600C gives nitrobenzene.

3. Sulphonation : When benzene is heated with conc. H2SO4 , benzene sulphonic acid is formed.

4. Friedel – Craft’s reaction :
- Friedel Craft’s alkylation : Introduction of an alkyl group ( – R ) in the benzene ring by treating benzene with an alkyl halide (R-Cl or R-Br) in the presence of anhydrous AlCl3 is known as Friedel- Craft’s alkylation. Eg.
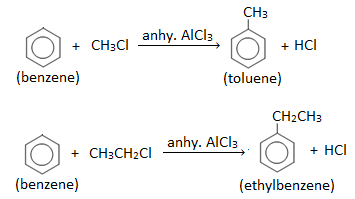
- Friedel Craft’s acylation : Introduction of an acyl group (i.e. keto group) ( RCO- ) in the benzene ring by treating benzene with an acylating agent like acid chloride (RCOCl) or acid anhydride in the presence of anhydrous AlCl3 is known as Friedel- Craft’s acylation. Eg.


Similarly, benzene when treated with benzoyl chloride in the presence of anhydrous AlCl3 gives benzophenone.

Addition reactions of benzene
Because of unusual stability of benzene ring( due to delocalization of π – electrons), addition reactions are difficult to take place. However due to presence of three double bonds, under proper condition (drastic condition)(i.e high temperature and pressure) addition reaction takes place.
- Addition of hydrogen : When benzene vapour is heated with hydrogen gas in presence of nickel or platinum catalyst, cyclohexane is formed.
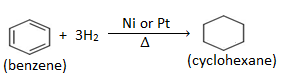
- Addition of halogen : Benzene adds three molecules of chlorine in presence of sunlight ( UV light) to give benzene hexachloride (BHC).

BHC is extensively used in agriculture as pesticide under the trade name gammexane or lindane or 666.
Combustion reaction of benzene
Benzene burns in air with sooty flame to give carbondioxide and water.
![]()
Uses of benzene
- It is used as a starting material for the preparation of varieties of aromatic compounds which are used for the manufacture of dyes, drugs, perfumes, explosives, etc. ( Eg. benzene is used for making toluene which is needed for making TNT.)
- It is used as a solvent for the extraction of fat and oil.
- It is used as a fuel for automobiles in the name of benzol. {It is used in gasoline to increase the octane rating of gasoline.}
- It is used for dry cleaning of woolen clothes.
- It is used for making phenol needed for producing Bakelite.
Benzene preparation and properties (reactions) flowchart
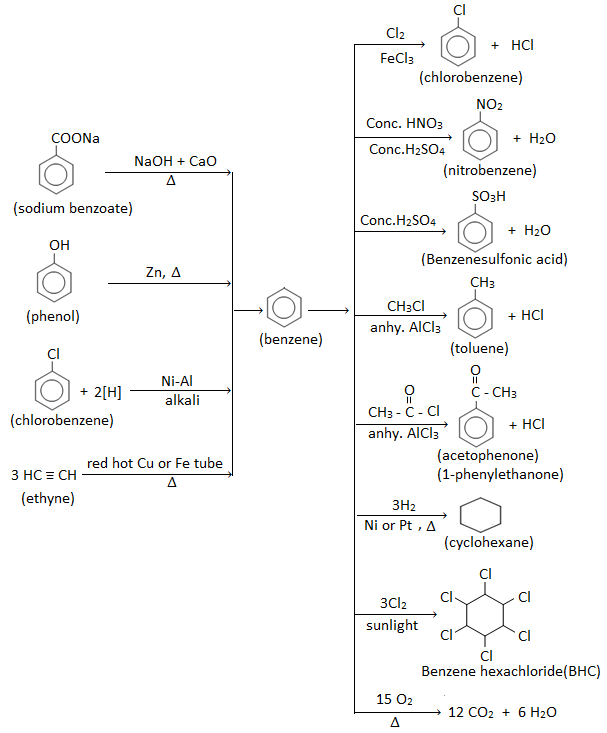
Questions and their answers
Q ) How will you convert:
1. Benzoic acid into benzene and vice-versa.
2. Phenol to toluene.
3. Phenol to acetophenone.
4. Toluene into benzene.
Answer :
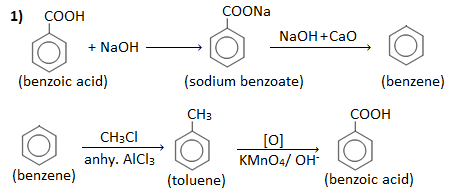
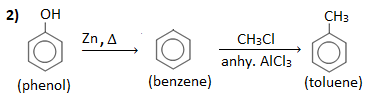


Q) Identify X and Y :

Answer :

Therefore, compound ‘X’ is benzene and compound ‘Y’ is toluene.
Q) Is cycloheptatriene an aromatic compound? Give reason.
 cycloheptatriene seems to follow Huckel’s rule i.e. 4n + 2 = 6 , or n = 1 but the π – electrons system is not delocalized due to presence of a sp3 hybridized carbon in the ring and hence does not behave as an aromatic compound.
cycloheptatriene seems to follow Huckel’s rule i.e. 4n + 2 = 6 , or n = 1 but the π – electrons system is not delocalized due to presence of a sp3 hybridized carbon in the ring and hence does not behave as an aromatic compound.
Q) Identify A to L.

References
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Morrison, R.T. , Boyd, R.N., Organic Chemistry, Sixth edition, Prentice-Hall of India Pvt. Ltd., 2008.
- https://pubchem.ncbi.nlm.nih.gov/compound/Benzene
- https://www.britannica.com/science/benzene
- https://www.chemguide.co.uk/basicorg/conventions/names3.html
Elimination reaction : E1 and E2 reaction – Examples, Mechanism, Orientation and Reactivity
Elimination reaction
An elimination reaction is a type of organic reaction in which a pair of atoms or group of atoms are removed from a organic molecule. Elimination reaction is the principal process by which saturated organic compounds (i.e compounds containing carbon – carbon single bonds) are converted to unsaturated organic compounds (i.e. compounds containing carbon – carbon double or triple bonds). For example- Dehydrohalogenation reaction of alkyl halides.
When an alkyl halide is heated with an alcoholic solution of KOH or NaOH, an alkene is formed by the elimination of a molecule of hydrogen halide. This reaction is known as dehydrohalogenation of alkyl halide. This reaction involves the removal of hydrogen atom together with the halogen atom from the adjacent carbon atom, so this reaction is also called the α,β- elimination reaction.

Types of elimination reactions
Elimination reaction is of two types:
- E2 reaction
- E1 reaction
E2 reaction
E2 reaction is also known as elimination bimolecular reaction. This reaction occurs when an alkyl halide is treated with a strong base such as hydroxide ion (OH-) and forms a carbon-carbon double bond. Example:

Kinetics of E2 reaction :
In E2 reaction, the rate of dehydrohalogenation (i.e. alkene formation) depends upon the concentration of both alkyl halide and base. It follows second order kinetics.
Rate α [Alkyl halide] [Base]
R = k[RX] [:B]
Mechanism of E2 reaction :
E2 mechanism is a one step process. Base attacks the hydrogen atom of β-carbon and begins to remove the H atom and at the same time as the carbon-carbon double bond starts to form, the –X group starts to leave as shown below in transition state. After the transition state, C-H bond and C-X bond are completely broken and carbon-carbon double bond is formed.

Energy profile diagram of E2 reaction:

Orientation and reactivity of E2 reaction:
In some cases, this reaction yields a single alkene but in other cases a mixture of alkenes are formed. For example, 2-bromopropane forms only propene whereas 2-bromobutane forms a mixture of 1-butene and 2-butene.


If there is a possibility of formation of mixture of alkenes, the E2 reaction follows Saytzeff rule. This rule states that the major product is the alkene that has greater number of alkyl groups attached to the double bonded carbon atoms. Therefore, the stability order of various alkenes is:
R2CH=CR2 > R2C=CHR > R2C=CH2 > RCH=CHR > RCH=CH2 > CH2=CH2
Hence, in dehydrohalogenation reaction, the more stable the alkene more easily or faster it is formed.
E1 reaction
E1 reaction is also known as elimination unimolecular reaction. This reaction is particularly common in secondary and tertiary alkyl halides in absence of a strong base. For example, when 2-bromo-2-methylpropane is treated with aqueous ethanol, 2-methyl propene is formed.

Kinetics of E1 reaction:
In E1 reaction, the rate of alkene formation depends upon the concentration of alkyl halide only. It follows first order kinetics.
Rate α [Alkyl halide]
R = k [RX]
Mechanism of E1 reaction:
E1 mechanism is a two step process.
Step I : In this step the molecule of alkyl halide undergoes ionization to give a carbocation (carbonium ion) and halide ion.

Step II : In this step, the carbocation loses a proton from the adjacent carbon to yield the alkene.

In a chemical reaction the slow step is rate determining step, so first step is rate determining step of E1 reaction.
In certain alkyl halides, the carbocation initially formed undergoes rearrangement to form more stable carbocation and thus highly branched alkene is formed as the major product.
For example, dehydrohalogenation of 3-chloro-2,2-dimethylbutane yields 2,3-dimethylbut-2-ene as major product.

Energy profile diagram of E1 reaction:

Orientation and reactivity of E1 reaction:
Elimination by E1 reaction shows Saytzeff’s orientation. For example;

Reactivity of alkyl halide to E1 reaction is determined by the rate of formation of carbocation which in turn depends on stability of carbocation. Hence, order of reactivity of alkyl halides in E1 reaction is:
30 haloalkane > 20 haloalkane > 10 haloalkane
References
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Morrison, R.T. , Boyd, R.N., Organic Chemistry, Sixth edition, Prentice-Hall of India Pvt. Ltd., 2008.
- March, j., Advanced Organic Chemistry, Fourth edition, Wiley Eastern Ltd. India, 2005.
- https://www.britannica.com/science/elimination-reaction
Aldol condensation and Crossed aldol condensation reaction- Definition, Examples, Mechanism and Uses.
Contents [hide]
Aldol condensation reaction
Aldol condensation reaction is one of the important reactions of carbonyl compounds (i.e. aldehydes and ketones)
Condensation between two molecules of aldehydes or ketones having at least one α – hydrogen atom in presence of dilute alkali to form β-hydroxy aldehyde or β-hydroxy ketone is known as aldol condensation reaction. Examples:
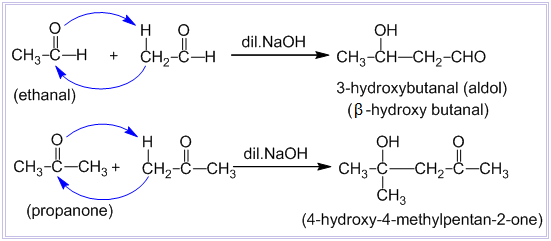
Aldehydes and ketones which do not contain any α – hydrogen atom such as HCHO, (CH3)3CCHO, C6H5CHO, etc. do not undergo aldol condensation reaction.
Mechanism of aldol condensation reaction
Taking acetaldehyde (ethanal) as an example, aldol condensation involves following steps:
Step-I :
In this step, hydroxide ion from alkali removes a proton from the α – carbon of one molecule of ethanal to give a carbanion (i.e. enolate ion).
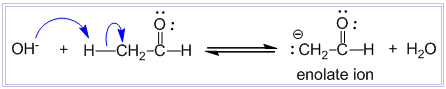
Step-II :
In this step, there is nucleophilic addition of enolate ion to the carbonyl carbon of second molecule of ethanal to produce an alkoxide ion.
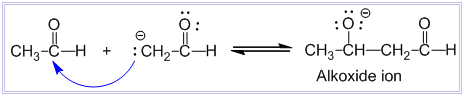
Step-III :
In this step, the alkoxide ion takes up a proton from water to form β-hydroxy aldehyde (aldol).

Dehydration of aldol products
The product of aldol condensation on heating with dilute acids undergo dehydration to form α, β-unsaturated aldehydes or ketones. Examples:

Uses of aldol condensation in synthesis
Aldol products contain two functional groups (-OH and –CHO) and hence they can be used in a number of reactions to give various products. For example:
1. Catalytic hydrogenation of α, β-unsaturated aldehydes or ketones, obtained from dehydration of aldol products gives saturated alcohols.

2. Reduction of α, β-unsaturated aldehydes or ketones by LiAlH4 gives unsaturated alcohol.

Crossed aldol condensation reaction
- Aldol condensation between two different carbonyl compounds is called a crossed aldol condensation.
- Crossed aldol condensation is not useful in laboratories if both of the carbonyl compounds have α-hydrogen, because a mixture of products are formed.
- However, it is useful when one of the carbonyl compounds does not have α-hydrogen and therefore cannot undergo self condensation. For example: benzaldehyde can be used with other aldehydes and ketones containing α-hydrogen.

Mechanism of crossed-aldol condensation reaction
[same as that of aldol condensation reaction]
Taking acetaldehyde (ethanal) and benzaldehyde as examples of aldehydes, crossed aldol condensation involves following steps:
Step-I :
In this step, hydroxide ion from alkali removes a proton from the α – carbon of ethanal to give a carbanion (i.e. enolate ion).

Step-II :
In this step, there is nucleophilic addition of enolate ion to the carbonyl carbon of benzaldehyde to produce an alkoxide ion.
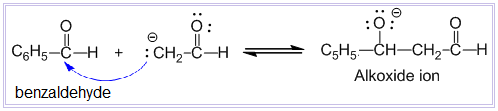
Step-III :
In this step, the alkoxide ion takes up a proton from water to form β-hydroxy aldehyde (aldol).

References
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Morrison, R.T. , Boyd, R.N., Organic Chemistry, Sixth edition, Prentice-Hall of India Pvt. Ltd., 2008.
- March, j., Advanced Organic Chemistry, Fourth edition, Wiley Eastern Ltd. India, 2005.
Aromatic Aldehydes and Ketones – Preparation and Properties
Contents [hide]
What are aromatic aldehydes and ketones?
Aromatic aldehydes are the compounds in which –CHO group is bonded directly to an aromatic ring. Eg.

Aromatic ketones are the compounds in which carbonyl group is bonded with either both aryl group or aryl and alkyl group. Eg.

Note: The compound in which carbonyl group is not bonded directly to the benzene ring are considered as arly substituted aliphatic aldehydes. Eg.

Preparation of benzaldehyde and acetophenone
Preparation of benzaldehyde:
1. From toluene: Benzaldehyde is prepared by oxidation of toluene with cerium oxide (CeO2) in the presence of conc. H2SO4.

2. From Rosenmund’s reduction : Benzaldehyde is obtained by reducing benzoyl chloride with hydrogen in the presence of Pd catalyst deposited in BaSO4.

Preparation of acetophenone from benzene:
Acetophenone is prepared by the treatment of benzene with acetyl chloride in the presence of anhydrous AlCl3.
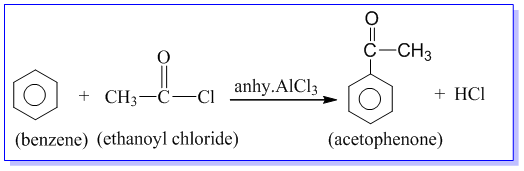
Properties of benzaldehyde
1. Cannizzaro’s reaction:
Aldehydes which do not contain α-hydrogen like HCHO, C6H5CHO,etc. undergo self oxidation and reduction on treatment with conc. alkali. In this reaction one molecule is oxidized to carboxylic acid and other molecule is reduced to alcohol. Thus, a mixture of an alcohol and a salt of carboxylic acid is formed by Cannizzaro’s reaction.
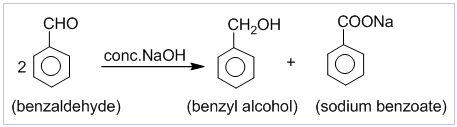
2. Perkin’s (condensation) reaction:
The condensation of an aromatic aldehyde with an acid anhydride in the presence of sodium or potassium salt of the same acid to produce α,β-unsaturated acid is known as the Perkin’s condensation.
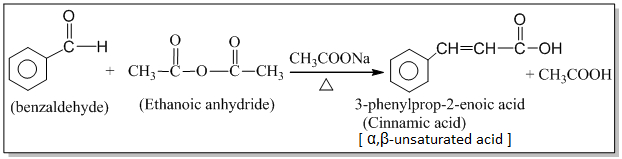
3. Benzoin condensation reaction:
Benzaldehyde when heated with alcoholic solution of potassium cyanide, undergoes self condensation between two molecules to form an α-hydroxy ketone known as benzoin. This reaction is called benzoin condensation reaction. Eg.
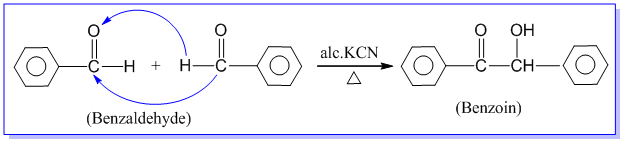
4. Electrophilic substitution reaction:
-CHO group is electron withdrawing group. It withdraws ∏- electrons from benzene ring, decreasing electron density of aromatic ring.
Benzaldehyde is resonance hybrid of following resonance structures:
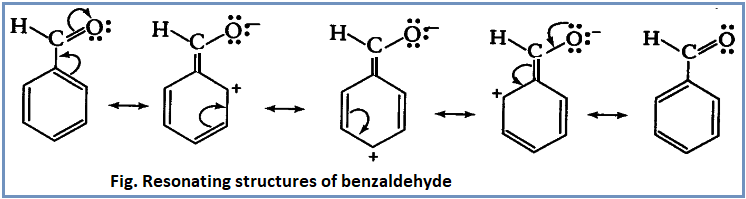
From the above resonating structures, it is clear that electron density is comparatively high at meta position. Thus incoming electrophile attacks at meta position to give meta substituted product. Thus –CHO is a meta directing group.

Similarly, acetophenone also undergoes electrophilic substitution reaction at meta position.

- Other reactions of aromatic aldehydes and ketones are similar to the reactions of aliphatic aldehydes and ketones.
- See the reactions of aliphatic aldehydes and ketones….
REFERENCES
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Morrison, R.T. , Boyd, R.N., Organic Chemistry, Sixth edition, Prentice-Hall of India Pvt. Ltd., 2008.
- https://www.snapsolve.com/class11/chemistry/cbse-1100177433
- https://chemicalnote.com/aldehydes-and-ketones-carbonyl-compounds-preparation-and-properties/
Electrophilic substitution reactions of benzene with mechanism.
The most important/common reactions of benzene are electrophilic substitution reactions. In electrophilic substitution reactions an electrophile attacks the benzene and substitutes one of the hydrogen atoms of benzene ring to give substituted product.

Electrophiles : (Electron lovers) . Electrophiles are electron deficient species which attack on electron rich centre during chemical reactions. These are either a positively charged species or the neutral species having electron deficient centre. Eg. Cl+, CH3+, SO3, AlCl3, BF3, etc.
General mechanism of electrophilic substitution reactions

Four types of electrophilic substitution reactions of benzene are described below with their mechanism.
Halogenation of benzene
Benzene reacts with halogen in presence of a Lewis acid such as FeCl3 to give halobenzene. For example, chlorine reacts with benzene in presence of ferric chloride or AlCl3 as catalyst to give chlorobenzene.

Similarly, benzene reacts with bromine in presence of ferric bromide as catalyst to give bromobenzene.

Mechanism of halogenation of benzene
This reaction involves the following steps:

Nitration of benzene
When benzene is heated with conc. HNO3 in the presence of conc. H2SO4 at about 600C gives nitrobenzene.

Mechanism of nitration of benzene
This reaction involves the following steps:

Sulphonation of benzene
When benzene is heated with conc. H2SO4 , benzene sulphonic acid is formed.

Mechanism of sulphonation of benzene
This reaction involves the following steps:

Friedel – Craft’s reaction of benzene
Friedel- Craft’s reaction is of two types- alkylation and acylation.
Friedel Craft’s alkylation reaction
Introduction of an alkyl group ( – R ) in the benzene ring by treating benzene with an alkyl halide (R-Cl or R-Br) in the presence of anhydrous AlCl3 is known as Friedel- Craft’s alkylation reaction. Eg.

Mechanism of Friedel-Craft’s alkylation reaction
This reaction involves the following steps:

Friedel craft’s acylation reaction
Introduction of an acyl group (i.e.keto group) (  ) in the benzene ring by treating benzene with an acylating agent like acid chloride (RCOCl) or acid anhydride in the presence of anhydrous AlCl3 is known as Friedel- Craft’s acylation reaction. Eg.
) in the benzene ring by treating benzene with an acylating agent like acid chloride (RCOCl) or acid anhydride in the presence of anhydrous AlCl3 is known as Friedel- Craft’s acylation reaction. Eg.

Similarly, benzene when treated with benzoyl chloride in the presence of anhydrous AlCl3 gives benzophenone. This reaction is also called benzoylation reaction.

Mechanism of Friedel-Craft’s acylation reaction:
This reaction involves the following steps:

References
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Sharma, M.L., Chaudary, P.N., A Text Book of B.Sc. Chemistry, Second edition (Volume one and two), Ekta Books, Kathmandu, 2004.
- Sthapit, M.K., Pradhananga, R.R., Foundations of Chemistry, Vol 1 and 2, Fourth edition, Taleju Prakashan, 2005.
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Arenes/Reactivity_of_Arenes/Substitution_Reactions_of_Benzene_Derivatives
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/friedel-crafts-reaction
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Reactions/Substitution_Reactions/Electrophilic_Substitution_Reactions/The_Halogenation_of_Benzene.
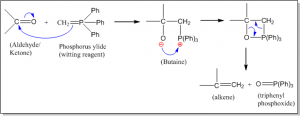





No comments:
Post a Comment