Carbonyl compounds [Aldehydes and ketones]
Structure and nature of the carbonyl group
Nomenclature of aldehydes and ketones
Isomerism in aldehydes and ketones
General methods of preparation of aldehydes and ketones
Physical Properties of aldehydes and ketones
Chemical Properties[A] Nucleophilic addition reaction
[B] Addition followed by elimination of water molecule [Addition of ammonia derivatives]
2,4-DNP test
[C] Oxidation reactions of aldehydes
[D] Haloform reaction
[E] Reduction reaction
[F] Special reactions of methanal (formaldehyde)
Aldol condensation reaction
Cannizzaro’s reaction
Formalin and its Uses
REFERENCES
Carbonyl compounds [Aldehydes and ketones]
Aldehydes and ketones are the compounds containing carbonyl group, so are collectively called carbonyl compounds.

Structure and nature of the carbonyl group
In carbonyl group, there is carbon to oxygen double bond, which consist of a sigma (σ) bond and a pi (π) bond
 .
.
Both of the carbon and oxygen atoms are sp2 hybridized. One sp2 hybrid orbital of carbon forms σ-bond with oxygen atom and remaining two hybrid orbitals form σ-bond with hydrogen or carbon atom. π -bond is formed by the overlap of unhybridized orbitals of carbon and oxygen atom.
This carbonyl group is trigonal and planar with bond angle of 1200.

In carbonyl group, carbon atom is bonded with oxygen atom which is more electronegative than carbon. Thus, the bonded pair of electrons lie more closer to the oxygen atom than carbon atom which leads to the polarization in carbon-oxygen bond. There is charge separation, oxygen atom acquires slightly negative charge while the carbon atom acquires slightly positive charge.

Nomenclature of aldehydes and ketones

Isomerism in aldehydes and ketones
1 . Chain Isomerism: Aldehydes having at least 4 carbon atoms and ketones having at least 5 carbon atoms show chain isomerism. Eg.

2. Position isomerism: Ketones having at least 5 carbon atoms and aromatic aldehydes show position isomerism.Eg.

3. Functional isomerism: Ketone and aldehyde having same molecular formula are functional isomers of one another.

4. Metamerism: Ketones exhibit metamerism due to difference in alkyl group present on either side of carbonyl group.
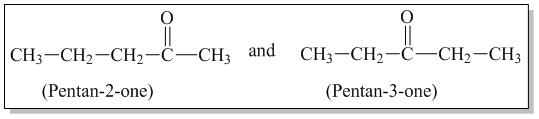
Q) Write the possible isomeric aldehydes and ketones that can be formed from C4H8O.
Ans.
General methods of preparation of aldehydes and ketones
1. From alcohol:
(i) By oxidation of alcohols:
- Alcohols on controlled oxidation give aldehydes and ketones.
- Acidified KMnO4 or K2Cr2O7 is used as oxidizing agent.
- 10 alcohol gives aldehyde while 20 alcohol gives ketone. Eg.

(ii) By dehydrogenation of alcohols:
When alcohol vapors are passed over heated copper at 3000C, different types of alcohols give different products.
- Primary alcohols are dehydrogenated to aldehydes. Eg.

- Secondary alcohols are dehydrogenated to ketones. Eg.

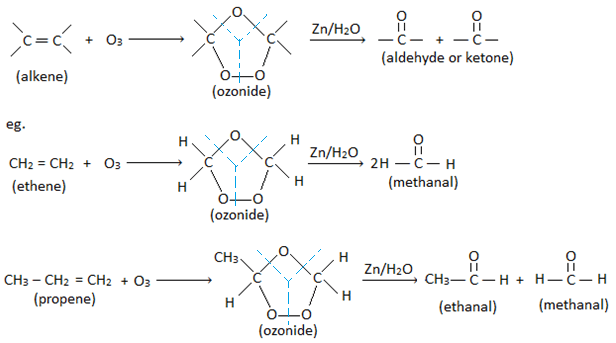
2. By ozonolysis of alkene:
Alkene reacts with ozone to give ozonide. On warming ozonide with Zn in water, it breaks down to give two molecules of carbonyl compounds (aldehyde or ketone). This process of formation of ozonide and it’s decomposition to give carbonyl compounds is called ozonolysis.
3. By catalytic hydration of alkynes :
Alkynes react with water in presence of mercuric sulphate and sulphuric acid to give vinyl alcohol which rearranges to give aldehyde or ketone.

For example, ethyne gives ethanal (i.e. aldehyde).

Propyne gives propanone (i.e. ketone).

4. From acid chlorides:
(i) By Rosenmund reduction: Aldehydes can be prepared by reducing acid chloride solution with hydrogen in the presence of Palladium(Pd) catalyst deposited on barium sulphate and partially poisoned with sulphur or quinoline. This reaction is called Rosenmund reduction.

(ii) Ketones can be prepared by treating acid chloride with dialkyl cadmium.

Q) How would you convert benzoic acid into benzaldehyde?

5. From gem-dihalides:
The alkaline hydrolysis of gem-dihalide gives aldehyde and ketone.
Aldehydes are formed when two halogen atoms are attached to terminal carbon atom.
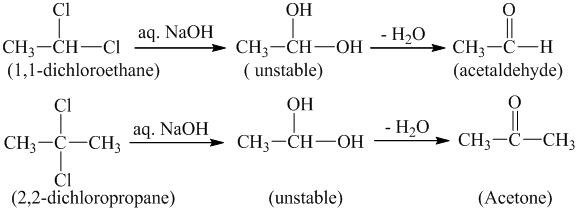
Ketones are formed when two halogen atoms are attached to non-terminal carbon atom.
Physical Properties of aldehydes and ketones
1. Boiling point: Aldehydes and ketones have higher boiling point than hydrocarbon of comparable molecular masses. This is because aldehydes and ketones contain polar carbonyl group and therefore there exists strong dipole-dipole interaction between the opposite end of C=O dipoles.

However, aldehydes and ketones have lower boiling point than alcohols and carboxylic acid of comparable molecular masses. This is because dipole-dipole interaction is weaker than intermolecular H-bonding.
2. Solubility: Lower aldehydes and ketones containing up to 4 carbon atoms are soluble in water due to formation of hydrogen bond between the polar carbonyl group and water molecule.

Chemical Properties
[A] Nucleophilic addition reaction
Aldehydes and ketones undergo nucleophilic addition reaction due to presence of polar carbonyl group.

The positively charged carbon of carbonyl group is readily attacked by nucleophilic species for the initiation of reaction. This leads to the formation of intermediate anion which is then attacked by electrophile (eg.H+) to give the final addition product.
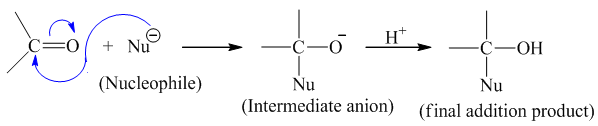
Q) Why does aldehydes easily undergoes nucleophilic addition reaction as compared to that of ketone?
Aldehydes and ketones contain polar carbonyl group and hence carbonyl carbonyl carbon is a suitable site for nucleophilic attack.

In aldehydes, one electron releasing alkyl group is attached to carbonyl carbon while in ketone two alkyl groups are attached to carbonyl carbon. Thus, aldehydic carbon is electron deficient than ketonic carbon and hence aldehyde is more easily attacked by nucleophilic species.
1. Addition of HCN: Aldehydes and ketones react with hydrogen cyanide to form addition product called cyanohydrins. Eg.

Cyanohydrin on acidic hydrolysis gives α-hydroxy acids which on heating loses a molecule of water to form α,β-unsaturated acid.

Q) Identify A, B , C and D.

Q) How would you obtain 2-hydroxy-2-methylpropanoic acid from propanone?
2. Addition of sodium bisulphite: Aldehydes and ketones react with saturated solution of sodium bisulphite to form crystalline bisulphite addition products. Eg.

3. Addition of Grignard reagent:
Aldehydes and ketones (i.e carbonyl compounds) when treated with Grignard reagent gives addition product, which on acidic hydrolysis give alcohols.

⊗ Formaldehyde gives primary alcohol. Eg.

⊗ Aldehydes other than formaldehyde give secondary alcohol. Eg.

⊗ Ketones give tertiary alcohol. Eg.

[B] Addition followed by elimination of water molecule [Addition of ammonia derivatives]
Aldehydes and ketones react with number of ammonia derivatives such as hydroxylamione(NH2OH), hydrazine(NH2-NH2), phenylhydrazine(C6H5NHNH2), etc. in weakly acidic medium to form compounds containing C=N group.
1. Reaction with hydroxylamine: Aldehydes and ketones react with hydroxylamine to form oximes. Eg.
![Addition followed by elimination of water molecule [Addition of ammonia derivatives]](https://chemicalnote.com/wp-content/uploads/2022/02/word-image-32.png)
2. Reaction with hydrazine: Aldehydes and ketones react with hydrazine to form hydrazone. Eg.

3. Reaction with phenyl hydrazine: Aldehydes and ketones react with phenyl hydrazine to form phenylhydrazones. Eg.
![Addition followed by elimination of water molecule [Addition of ammonia derivatives]](https://chemicalnote.com/wp-content/uploads/2022/02/word-image-34.png)
4. Reaction with 2,4-dinitrophenyl hydrazine : Aldehydes and ketones react with 2,4-dinitrophenyl hydrazine (2,4-DNP) to form yellow, orange or red ppt. of 2,4-dinitrophenyl hydrazone. Eg.

5. Reaction with semicarbazide: Aldehydes and ketones react with semicarbazide to form semicarbazone. Eg.
![Addition followed by elimination of water molecule [Addition of ammonia derivatives]](https://chemicalnote.com/wp-content/uploads/2022/02/word-image-36.png)
2,4-DNP test
Aldehydes and ketones react with 2,4-dinitrophenyl hydrazine (2,4-DNP) to form yellow, orange or red ppt. of 2,4-dinitrophenyl hydrazone. Eg.

Q) Write a chemical test to distinguish ethanal from ethanol?
Ethanal and ethanol can be distinguished by 2,4-DNP test. Ethanal (i.e aldehyde) reacts with 2,4-dinitrophenyl hydrazine (2,4-DNP) to form orange ppt. of ethanal 2,4-dinitrophenyl hydrazone. But ethanol does not give this test.

Note : 2,4-DNP = Brady’s reagent.
≠ Action with PCl5:
Aldehydes and ketones react with PCl5 to give gem-dichloroalkane (gem-dihalide).

[C] Oxidation reactions of aldehydes
Aldehydes are oxidized not only by strong oxidizing agents like KMnO4 or K2Cr2O7 but also by weak oxidizing agents like Br2 water, Ag+, Cu++, etc. So, aldehydes are strong reducing agents.
1. Reaction with Tollen’s reagent: [Silver mirror test]
Tollen’s reagent is an ammonical solution of silver nitrate. It is prepared by adding dilute solution of NH4OH to AgNO3 solution till the precipitate of Ag2O once formed gets dissolved.

Aldehydes on heating with Tollen’s reagent reduces the reagent to metallic silver.
![Reaction with Tollen’s reagent: [Silver mirror test]](https://chemicalnote.com/wp-content/uploads/2022/02/word-image-41.png)
The silver deposits on the inner wall of test tube forming a shining layer like mirror. Hence, this test is known as silver mirror test.
- Both aliphatic and aromatic aldehydes give this test but ketones do not give this test.
Q) Write the functional isomer of C3H6O and give a chemical test to distinguish them.
The functional isomers of C3H6O are:

These two isomers can be distinguished by silver mirror test (i.e. Tollen’s reagent). Propanal (i.e. aldehyde) gives positive silver mirror test but propanone (i.e. ketone) does not give this test.

2. Reaction with Fehling’s solution: [Fehling’s test]
Fehling’s solution is an alkaline solution of CuSO4 containing some Rochelle salt (i.e. sodium potassium tartarate. It is prepared by adding alkaline solution of Rochelle salt [Fehling solution B] to CuSO4 solution [Fehling solution A]. When an aliphatic aldehyde is heated with Fehling’s solution, a brick red ppt. of cuprous oxide is formed. This reaction is known as Fehling’s test.
![Reaction with Fehling’s solution: [Fehling’s test]](https://chemicalnote.com/wp-content/uploads/2022/02/word-image-44.png)
[D] Haloform reaction
Aldehydes and ketones containing CH3CO- group on reaction with excess halogen in presence of NaOH gives haloform (chloroform’ bromoform, iodoform). Eg.

Iodoform test :

Note:
- This reaction occurs in same way as lab preparation of chloroform. Eg.

- This reaction is used to distinguish some of the pairs of compounds. Eg.
- Ethanol and methanol
- Ethanal and methanal
- 2-pentanone and 3-pentanone, etc.
Q) How can you distinguish 2-pentanone and 3-pentanone.
2-pentanone and 3-pentanone can be distinguished by iodoform test as 2-pentanone gives iodoform reaction but 3-pentanone doesn’t give iodoform reaction. Eg.

[E] Reduction reaction
1. Reduction to alcohols: Aldehydes and ketones are reduced to primary and secondary alcohols respectively using H2 in presence of Ni, Pt, Pd or LiAlH4. Eg.


2 . Clemmensen’s reduction: The reduction of aldehydes and ketones to alkane using zinc amalgam and conc. HCl is Clemmensen’s reduction. In this reaction, carbonyl group (-CO-) is reduced to methylene group (-CH2-). Eg.

3 . Wolff-Kishner reduction : In this method aldehyde and ketone is treated with hydrazine to form hydrazone which is then heated with KOH in presence of glycol to give alkane. Eg.

4 . Reduction with HI in presence of red P : Aldehydes and ketones can be reduced into corresponding hydrocarbon when heated with HI in presence of red phosphorus at 1500C.

[F] Special reactions of methanal (formaldehyde)
1. Reaction with ammonia: Formaldehyde reacts with ammonia to form hexamethylene tetramine which is commonly known as ‘urotropine’. It is used as medicine to treat urinary infections.

2. Reaction with phenol:
Phenol condenses with formaldehyde in the presence of an acid or basic catalyst to form a polymer called Bakelite.

Formation of linear polymer:

Formation of cross-linked polymers:

Aldol condensation reaction
Condensation between two molecules of aldehydes or ketones having at least one α – hydrogen atom in presence of dilute alkali to form β-hydroxy aldehyde or β-hydroxy ketone is known as aldol condensation reaction. Examples:

Aldehydes and ketones which do not contain any α – hydrogen atom such as HCHO, (CH3)3CCHO, C6H5CHO, etc. do not undergo aldol condensation reaction.
Note : Dehydration of aldol product gives α, β-unsaturated aldehyde or ketone.

Cannizzaro’s reaction
Aldehydes which do not contain α-hydrogen like HCHO, C6H5CHO,etc. undergo self oxidation and reduction on treatment with conc. alkali. In this reaction one molecule is oxidized to carboxylic acid and other molecule is reduced to alcohol. Thus, a mixture of an alcohol and a salt of carboxylic acid is formed by Cannizzaro’s reaction
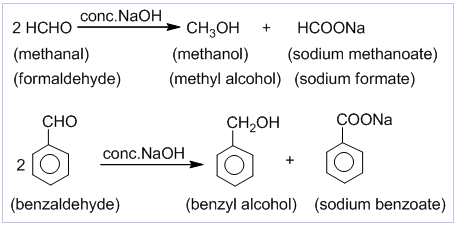
Formalin and its Uses
A 37-40% solution of formaldehyde in water is called formalin. Its molecular formula is HCHO (i.e. formaldehyde).
Uses of Formalin:
- It is used in preservation of biological specimens.
- It is used as an antiseptic and disinfectant.
- It is used to manufacture urinary antiseptic i.e. Urotropin.
- It is used to manufacture polymers like Bakelite, resins, etc.
- It is used in the manufacture of dyes like indigo, pararosaniline, etc.
REFERENCES
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Morrison, R.T. , Boyd, R.N., Organic Chemistry, Sixth edition, Prentice-Hall of India Pvt. Ltd., 2008.
- March, j., Advanced Organic Chemistry, Fourth edition, Wiley Eastern Ltd. India, 2005.
- https://chemicalnote.com/category/organic-chemistry/name-reactions/
- https://www.dailymotion.com/video/x3nq0aj
- https://www.rxlist.com/formalin/definition.htm
