Barium Carbonate
Introduction
Barium Carbonate has a molecular formula,
Introduction
Barium Carbonate has a molecular formula,
Introduction
Barium Carbonate has a molecular formula, Ba CO₃. It is used for the formation of rat poisons. It is also used in paints, ceramics, rubber, plastics, etc. industries. It is also used for the formation of refractive indices of the glasses.
What is Barium Carbonate?
Barium carbonate is an inorganic chemical compound with molecular or empirical formula, Ba CO₃. It is tasteless and odourless. It has a white salt appearance like most of the alkaline earth metal carbonates. It is poorly or insoluble in pure water but it is partially soluble in the water saturated with carbon dioxide
Images Coming soon
Physical Properties of Barium Carbonate
Following are the all possible physical properties of barium carbonate−
Solubility: As we have discussed in the above paragraphs that barium carbonate is insoluble in pure water but it is soluble in the water−saturated with Carbon dioxide
Specific Heat: It has a specific heat of around 0.1448.
Appearance: It generally appears as white crystals or white salt powder.
Complexity: It has an atom complexity of around 18.78.
Covalently-Bonded Unit: It has in total two covalently-bonded units.
Chemical Properties of Barium Carbonate
Following are some of the chemical properties of barium carbonate−
Calcium salts (these are soluble salts ) can easily react with the barium carbonate to form to give Barium sulphate that remains in the solution as precipitate and calcium carbonate. The chemical reaction for the same has been shown below:
- Barium carbonate can also react with hydrochloric acid to form or give Barium chloride Ba Cl2,Water H2O
and carbon dioxide
Barium Carbonate Structure
This is a 3-D representation of the Barium carbonate molecule.
Images Coming soon
This is the empirical representation of the same barium carbonate that is a combination of
Images Coming soon
Various Uses of Barium Carbonate
Following are the all possible uses of Barium Carbonate -
As it is founded to be white insoluble salt, it is widely used in the Ceramics industry for the formation or generation of a wide variety of ceramic products.
It is also used as a raw material for the other barium products like Barium oxide
Barium carbonate is also used widely for the formation of rodenticide (rat poison). Due to its whitish salt-like or flour-like appearance, it attracts rats towards it.
Some other major commercial uses or applications of barium carbonate are for the formation of the refractive index of the glasses, in oil-drilling industries, photography, enamel formation, magnetic materials formation, paint and brick formation and in a large number of chemical industries.
It also plays a major role in the manufacturing of electronic ceramics (which are used in a large amount nowadays), PTC thermistors, capacitors and various other types of electronic equipment.
Barium carbonate also plays an important role in the production or generation of magnetic components and fibre optical glasses.
Production Method of Barium Carbonate
Various Production Methods for the formation of Barium carbonate are the Carbonation method, Metathesis method, Poison Nepheline Conversion method, Dry Granulation method and Wet Granulation method. Now, we will discuss each one of them one by one
Carbonation Method- The process for the production of
First of all carbon dioxide gas is passed through the solution of barium sulfide
Then the barium carbonate in the form of slurry will be obtained from this process and is then further subjected to desulphurization wash.
Further, it is passed through vacuum filtration and then dried at approx 300 degrees Celcius.
And, finally, a process called pulverization will take place before barium carbonate products can be obtained.
The chemical reaction involved here is
Poison Nepheline Conversion Method- Here, in this process soluble barium salt is generated by reacting witherite (a mineral consisting of barium carbonate) with an ammonium salt
Then the resultant ammonium carbonate is restored or recycled. This ammonium carbonate is further added to the soluble barium salt obtained earlier. The resultant barium carbonate is then filtered out and dried to obtain barium carbonate-based products.
The chemical reaction involved here is
Metathesis Method- In this method, barium sulfide
The chemical reaction involved here is
Wet Granulation Method- Here, one of the very known precipitation systems is used to filter to throw out a cake containing barium rich in water in the process of formation. The required material is then passed or moved through the action of rotating blades and the materials are rapidly mixed. It is then further kneaded or mixed to form semi-dense particles. These wet particles are then put into the rotary kiln’s direct fire and finally we get the particles of barium carbonate.
Dry Granulation Method- Here barium carbonate is obtained from the heavy precipitation(exceeds normal amount) that is seived and placed within the warehouse(place to store raw materials) of raw materials.
Conclusion
Here, in this article, we come to know about barium carbonate which is an inorganic chemical compound that occurs in white crystals or as a whitish salt powder. It is colourless and odourless. It is insoluble in pure water but soluble in the water-saturated with some amount of carbon dioxide and it is also soluble in most of the acids except sulphuric acid. Further, I discussed the physical and chemical properties of barium carbonate which include its solubility, complexity, specific heat, covalently-bonded atoms, and capability of reacting with other metals or salts. We have also gone through the structure of barium carbonate, its various uses and all the possible methods for its formation briefly.
FAQs
Q1. What is Barium Carbonate?
Barium Carbonate is an inorganic chemical compound, with molecular or empirical formula
Q2. Barium carbonate contains how many covalently-bonded units?
Barium carbonate contains in total two covalently-bonded units.
Q3. What do you mean by covalently bonded units?
Units or atoms formed due to the formation of covalent bonds which are formed by the equal sharing of electrons from both the participating atoms are termed covalently bonded units.
Q4. Mention some uses of Barium Carbonate.
Barium carbonate finds several uses in the commercial industries. And, some of them are ceramics, paints, bricks, rat poisons, oil drilling, magnetic components formation and many others.
Q5. What are the methods used for the production of Barium Carbonate?
Following are some of the major methods used for the formation of Barium Carbonate-
- Carbonation method
- Metathesis method
- Poison Nepheline Conversion method
- Dry Granulation method
- Wet Granulation method
What is Barium Carbonate?
Barium carbonate is an inorganic chemical compound with molecular or empirical formula,
Images Coming soon
Physical Properties of Barium Carbonate
Following are the all possible physical properties of barium carbonate−
Solubility: As we have discussed in the above paragraphs that barium carbonate is insoluble in pure water but it is soluble in the water−saturated with Carbon dioxide
Specific Heat: It has a specific heat of around 0.1448.
Appearance: It generally appears as white crystals or white salt powder.
Complexity: It has an atom complexity of around 18.78.
Covalently-Bonded Unit: It has in total two covalently-bonded units.
Chemical Properties of Barium Carbonate
Following are some of the chemical properties of barium carbonate−
Calcium salts (these are soluble salts ) can easily react with the barium carbonate to form to give Barium sulphate that remains in the solution as precipitate and calcium carbonate. The chemical reaction for the same has been shown below:
Barium carbonate can also react with hydrochloric acid
Barium Carbonate Structure
This is a 3-D representation of the Barium carbonate molecule.
Images Coming soon
This is the empirical representation of the same barium carbonate that is a combination of
Images Coming soon
Various Uses of Barium Carbonate
Following are the all possible uses of Barium Carbonate -
As it is founded to be white insoluble salt, it is widely used in the Ceramics industry for the formation or generation of a wide variety of ceramic products.
It is also used as a raw material for the other barium products like Barium oxide
Barium carbonate is also used widely for the formation of rodenticide (rat poison). Due to its whitish salt-like or flour-like appearance, it attracts rats towards it.
Some other major commercial uses or applications of barium carbonate are for the formation of the refractive index of the glasses, in oil-drilling industries, photography, enamel formation, magnetic materials formation, paint and brick formation and in a large number of chemical industries.
It also plays a major role in the manufacturing of electronic ceramics (which are used in a large amount nowadays), PTC thermistors, capacitors and various other types of electronic equipment.
Barium carbonate also plays an important role in the production or generation of magnetic components and fibre optical glasses.
Production Method of Barium Carbonate
Various Production Methods for the formation of Barium carbonate are the Carbonation method, Metathesis method, Poison Nepheline Conversion method, Dry Granulation method and Wet Granulation method. Now, we will discuss each one of them one by one
Carbonation Method- The process for the production of
First of all carbon dioxide gas is passed through the solution of barium sulfide
Then the barium carbonate in the form of slurry will be obtained from this process and is then further subjected to desulphurization wash.
Further, it is passed through vacuum filtration and then dried at approx 300 degrees Celcius.
And, finally, a process called pulverization will take place before barium carbonate products can be obtained.
The chemical reaction involved here is
Poison Nepheline Conversion Method- Here, in this process soluble barium salt is generated by reacting witherite (a mineral consisting of barium carbonate) with an ammonium salt
Then the resultant ammonium carbonate is restored or recycled. This ammonium carbonate is further added to the soluble barium salt obtained earlier. The resultant barium carbonate is then filtered out and dried to obtain barium carbonate-based products.
The chemical reaction involved here is
Metathesis Method- In this method, barium sulfide
The chemical reaction involved here is
Wet Granulation Method- Here, one of the very known precipitation systems is used to filter to throw out a cake containing barium rich in water in the process of formation. The required material is then passed or moved through the action of rotating blades and the materials are rapidly mixed. It is then further kneaded or mixed to form semi-dense particles. These wet particles are then put into the rotary kiln’s direct fire and finally we get the particles of barium carbonate.
Dry Granulation Method- Here barium carbonate is obtained from the heavy precipitation(exceeds normal amount) that is seived and placed within the warehouse(place to store raw materials) of raw materials.
Conclusion
Here, in this article, we come to know about barium carbonate which is an inorganic chemical compound that occurs in white crystals or as a whitish salt powder. It is colourless and odourless. It is insoluble in pure water but soluble in the water-saturated with some amount of carbon dioxide and it is also soluble in most of the acids except sulphuric acid. Further, I discussed the physical and chemical properties of barium carbonate which include its solubility, complexity, specific heat, covalently-bonded atoms, and capability of reacting with other metals or salts. We have also gone through the structure of barium carbonate, its various uses and all the possible methods for its formation briefly.
FAQs
Q1. What is Barium Carbonate?
Barium Carbonate is an inorganic chemical compound, with molecular or empirical formula
Q2. Barium carbonate contains how many covalently-bonded units?
Barium carbonate contains in total two covalently-bonded units.
Q3. What do you mean by covalently bonded units?
Units or atoms formed due to the formation of covalent bonds which are formed by the equal sharing of electrons from both the participating atoms are termed covalently bonded units.
Q4. Mention some uses of Barium Carbonate.
Barium carbonate finds several uses in the commercial industries. And, some of them are ceramics, paints, bricks, rat poisons, oil drilling, magnetic components formation and many others.
Q5. What are the methods used for the production of Barium Carbonate?
Following are some of the major methods used for the formation of Barium Carbonate-
- Carbonation method
- Metathesis method
- Poison Nepheline Conversion method
- Dry Granulation method
- Wet Granulation method
. It is an inorganic chemical compound. It is tasteless and odourless. Like or similar to most of the other alkaline earth metal carbonates, it appears as white salt and it is insoluble or poorly soluble in water solution but, it is soluble in most of the acids, except sulphuric acid. It is one of the very important barium compounds commercially or economically. Although it is not soluble in pure water it is partially or slightly soluble in water saturated with carbon dioxide
What is Barium Carbonate?
Barium carbonate is an inorganic chemical compound with molecular or empirical formula,
Images Coming soon
Physical Properties of Barium Carbonate
Following are the all possible physical properties of barium carbonate−
Solubility: As we have discussed in the above paragraphs that barium carbonate is insoluble in pure water but it is soluble in the water−saturated with Carbon dioxide
Specific Heat: It has a specific heat of around 0.1448.
Appearance: It generally appears as white crystals or white salt powder.
Complexity: It has an atom complexity of around 18.78.
Covalently-Bonded Unit: It has in total two covalently-bonded units.
Chemical Properties of Barium Carbonate
Following are some of the chemical properties of barium carbonate−
Calcium salts (these are soluble salts ) can easily react with the barium carbonate to form to give Barium sulphate that remains in the solution as precipitate and calcium carbonate. The chemical reaction for the same has been shown below:
Barium carbonate can also react with hydrochloric acid
Barium Carbonate Structure
This is a 3-D representation of the Barium carbonate molecule.
Images Coming soon
This is the empirical representation of the same barium carbonate that is a combination of
Images Coming soon
Various Uses of Barium Carbonate
Following are the all possible uses of Barium Carbonate -
As it is founded to be white insoluble salt, it is widely used in the Ceramics industry for the formation or generation of a wide variety of ceramic products.
It is also used as a raw material for the other barium products like Barium oxide
Barium carbonate is also used widely for the formation of rodenticide (rat poison). Due to its whitish salt-like or flour-like appearance, it attracts rats towards it.
Some other major commercial uses or applications of barium carbonate are for the formation of the refractive index of the glasses, in oil-drilling industries, photography, enamel formation, magnetic materials formation, paint and brick formation and in a large number of chemical industries.
It also plays a major role in the manufacturing of electronic ceramics (which are used in a large amount nowadays), PTC thermistors, capacitors and various other types of electronic equipment.
Barium carbonate also plays an important role in the production or generation of magnetic components and fibre optical glasses.
Production Method of Barium Carbonate
Various Production Methods for the formation of Barium carbonate are the Carbonation method, Metathesis method, Poison Nepheline Conversion method, Dry Granulation method and Wet Granulation method. Now, we will discuss each one of them one by one
Carbonation Method- The process for the production of
First of all carbon dioxide gas is passed through the solution of barium sulfide
Then the barium carbonate in the form of slurry will be obtained from this process and is then further subjected to desulphurization wash.
Further, it is passed through vacuum filtration and then dried at approx 300 degrees Celcius.
And, finally, a process called pulverization will take place before barium carbonate products can be obtained.
The chemical reaction involved here is
Poison Nepheline Conversion Method- Here, in this process soluble barium salt is generated by reacting witherite (a mineral consisting of barium carbonate) with an ammonium salt
Then the resultant ammonium carbonate is restored or recycled. This ammonium carbonate is further added to the soluble barium salt obtained earlier. The resultant barium carbonate is then filtered out and dried to obtain barium carbonate-based products.
The chemical reaction involved here is
Metathesis Method- In this method, barium sulfide
The chemical reaction involved here is
Wet Granulation Method- Here, one of the very known precipitation systems is used to filter to throw out a cake containing barium rich in water in the process of formation. The required material is then passed or moved through the action of rotating blades and the materials are rapidly mixed. It is then further kneaded or mixed to form semi-dense particles. These wet particles are then put into the rotary kiln’s direct fire and finally we get the particles of barium carbonate.
Dry Granulation Method- Here barium carbonate is obtained from the heavy precipitation(exceeds normal amount) that is seived and placed within the warehouse(place to store raw materials) of raw materials.
Conclusion
Here, in this article, we come to know about barium carbonate which is an inorganic chemical compound that occurs in white crystals or as a whitish salt powder. It is colourless and odourless. It is insoluble in pure water but soluble in the water-saturated with some amount of carbon dioxide and it is also soluble in most of the acids except sulphuric acid. Further, I discussed the physical and chemical properties of barium carbonate which include its solubility, complexity, specific heat, covalently-bonded atoms, and capability of reacting with other metals or salts. We have also gone through the structure of barium carbonate, its various uses and all the possible methods for its formation briefly.
FAQs
Q1. What is Barium Carbonate?
Barium Carbonate is an inorganic chemical compound, with molecular or empirical formula
Q2. Barium carbonate contains how many covalently-bonded units?
Barium carbonate contains in total two covalently-bonded units.
Q3. What do you mean by covalently bonded units?
Units or atoms formed due to the formation of covalent bonds which are formed by the equal sharing of electrons from both the participating atoms are termed covalently bonded units.
Q4. Mention some uses of Barium Carbonate.
Barium carbonate finds several uses in the commercial industries. And, some of them are ceramics, paints, bricks, rat poisons, oil drilling, magnetic components formation and many others.
Q5. What are the methods used for the production of Barium Carbonate?
Following are some of the major methods used for the formation of Barium Carbonate-
- Carbonation method
- Metathesis method
- Poison Nepheline Conversion method
- Dry Granulation method
- Wet Granulation method
References
A Process for the Purification of Barium Carbonate. - Patent GB ... Retrieved 2022, from https://pubchem.ncbi.nlm.nih.gov/patent/GB-190930339-A
Barium carbonate Definition & Meaning - Merriam-Webster. Retrieved 2022, from https://www.merriam-webster.com/dictionary/barium%20carbonate
Chemosensors | Free Full-Text | Promising Novel Barium Carbonate One ... Retrieved 2022, from https://www.mdpi.com/2227-9040/10/6/230/htm
The effect of PVP: BaTiO3 interlayer on the conduction mechanism and ... Retrieved 2022, from https://iopscience.iop.org/article/10.1088/1402-4896/abeba8
Barium Bromide
Introduction
The compound barium bromide is constituted by a metal and a non-metal element. Therefore, it is an ionic compound. The technique of fractional crystallization developed by Marie Curie in the 19th century to purify Radium employs barium bromide. Barium bromide is used in photography as a precursor of other important chemicals. Just like other halogen compounds, barium bromide has significant uses in inorganic chemistry. It is a chemical that is primarily used in laboratories and industries. Barium bromide is stored in glass bottles to prevent hydration. This is because barium bromide is a salt and can easily absorb water of hydration and turn it into its hydrated form.
What is Barium Bromide?
The reaction of a barium ion with a bromide ion produces a highly toxic ionic compound called barium bromide. Like any other ionic compound, barium bromide also exists in crystalline form. The three-dimensional lattice structure of barium bromide is orthorhombic. An orthorhombic structure is a crystal system that has two elongated orthogonal pairs that are stretched by two different factors. The overall crystal appears as a rectangular prism. Barium belongs to group 17 while barium belongs to group 2 which explains the ionic valency of both ions and stabilization of barium bromide attained by attaining a stable octet configuration.
Barium Bromide - Physical and Chemical Properties
Barium bromide is a white crystalline solid.
Barium bromide is deliquescent in nature. This means that it can adsorp or absorb molecules from the environment and hold it to form hydrated salts.
Barium bromide has a molecular weight of 297.14 g/mol
Barium bromide has a melting point of 857 degrees celsius. The reason for such a high melting point is that it is an ionic compound.
Barium bromide has a boiling point of 1835 degrees celsius. The reason for such a high boiling point is that it is an ionic compound.
Barium bromide has a density of 4.78 grams per cubic centimetre.
Barium bromide is soluble in water.
Reactions of Barium Bromide
Barium sulphate is precipitated out as a white solid when sulphate salts react with a solution of barium bromide. Sodium sulphate reacts with barium bromide via a double displacement reaction to produce barium sulphate.
Such a double displacement reaction of barium bromide can occur with other acids such as phosphoric acid, oxalic acid and hydrofluoric acid. These reactions are illustrated below.
The reaction of barium bromide with oxalic acid or any oxalate-producing compound yields barium oxalate as a solid precipitate.
The reaction of barium bromide with phosphoric acid or any phosphate-producing compound yield barium phosphate as a solid precipitate.
The reaction of barium bromide with hydrofluoric acid or any fluorine ion-producing compound yields barium fluoride as a solid precipitate.
Barium Bromide - How is the Compound Formed?
Generally, in laboratories barium bromide is extracted from its solution by the process of crystallization. This is done by heating the hydrated salt to 120 degrees celsius. This results in loss of water of hydration and anhydrous barium bromide formed as powdered solid. Other methods of synthesizing barium bromide are listed below.
The reaction of hydrobromic acid with barium sulphide produces a white solid precipitate named barium bromide.
The reaction of hydrobromic acid with barium carbonate produces a white solid precipitate named barium bromide.
How is Barium Bromide Used in Chemistry?
Barium bromide has various applications in laboratories and industries.
Barium bromide contributes to the formation of photographs as it helps in the production of compounds necessary for photo production.
Barium bromide was used to precipitate radium during the process of fractional crystallization.
Barium bromide being an ionic compound is used as a precursor in process of formation of other bromides.
Although it is less widely used than barium chloride, it is highly preferred in the formation of phosphorus.
It is also widely used to synthesize barium phosphate. Barium phosphate is an industrially important inflammable compound and is used in forming pulsed lasers and glasses.
Barium Bromide - Health Hazards
Barium bromide is a harmful toxic substance. This is the case for all water-soluble barium salts
Not just the solid form of barium bromide, but fumes of barium bromide can cause severe harm to health.
Exposure to barium bromide fumes may cause nausea, vomiting and discomfort for individuals and can lead to kidney, spleen or liver damage.
Barium bromide must not be swallowed as it is poisonous and may even lead to death.
Barium bromide can irritate the eyes and skin.
The inhalation of barium bromide fumes can lead to severe respiratory tract irritation and asphyxiation.
Since this salt is soluble in water it ionises into barium and bromide both of which can cause life-threatening damage to the human body.
Barium Bromide - Safety
Considering the harmful effects of barium bromide on the human body, one should follow proper preventive measures to avert severe injuries or damage.
In laboratories, barium bromide must be handled carefully. One should wear proper protective equipment such as gloves, lab coats, goggles, etc.
After handling and using barium bromide, it is recommended to wash the face, hand or any exposed part of the skin thoroughly.
Actions such as eating or drinking must be avoided when working with barium bromide.
One should keep the compound at a safe distance from their mouth and nose to avoid inhalation of any harmful fumes.
It must be ensured that the working area is properly ventilated to prevent suffocation.
The compound must be stored in an air-tight container to prevent contact with moisture, acids, strong oxidising agent or any other reactive substance.
Conclusion
In inorganic chemistry, barium bromide is an important alkaline halide. Barium bromide is used in the synthesis of various economically important compounds. Barium bromide shows almost every characteristic of a typical ionic compound. The solubility of this salt in the water makes it highly toxic to the human body. Since this compound is hygroscopic and can react with dust it should be handled with utmost care. This compound harms living tissues which is the reason this compound has more safety measures than applications. The poisonous and harmful nature of this salt limits the use of this solid in industries and laboratories.
FAQs
Q1. What happens if barium bromide is swallowed?
Barium bromide ionizes into barium ions and bromide ions when it comes in contact with water. Barium ions can act as poison for muscle stimulation which can lead to paralysis.
Q2. What first aid measures should be employed in case of exposure to barium bromide?
In case of exposure to barium bromide (inhaled or swallowed) a doctor or physician must be consulted as a priority.
The exposed area such as eyes or skin must be rinsed under water for a few minutes.
In case of inhalation, the affected individual should be taken to a well-ventilated area to avoid suffocation and breathlessness.
Q3. How can we drive the formula of barium bromide?
Barium is present as divalent ion –
Barium is present as monovalent ion –
Therefore, the formula is derived by the criss-cross method.
Images Coming soon
Q4. How does barium bromide exist as a coordination compound?
Barium bromide can exist as (distorted tetrahedral) and (trigonal pyramidal).
Q5. How can we identify the orthorhombic crystal system of barium bromide?
An orthorhombic system has 90-degree angles between all three axis The height, width and breadth of this system are unequal(a b c).
Images Coming soon
References
Brackett, E., Brackett, T., & Sass, R. (1963). THE CRYSTAL STRUCTURES OF BARIUM CHLORIDE, BARIUM BROMIDE, AND BARIUM IODIDE. The Journal Of Physical Chemistry, 67(10), 2132-2135. https://doi.org/10.1021/j100804a038
McGrath, J., & Silvidi, A. (1960). Proton-Magnetic-Resonance Study of Barium Bromide Monohydrate. The Journal Of Chemical Physics, 33(3), 644-647. https://doi.org/10.1063/1.1731230
McGrath, J., & Silvidi, A. (1962). Quadrupolar Study of Barium in Barium Bromide Dihydrate by Proton Magnetic Resonance. The Journal Of Chemical Physics, 36(6), 1496-1499. https://doi.org/10.1063/1.1732769
EMBLEM, H., & HARGREAVES, K. (2010). ChemInform Abstract: Preparation, Properties and Uses of Barium Compounds. Cheminform, 27(11), no-no. https://doi.org/10.1002/chin.199611322
Emblem, H., & Hargreaves, K. (1995). Preparation, Properties and Uses of Barium Compounds. Reviews In Inorganic Chemistry, 15(1-2).https://doi.org/10.1515/revic.1995.15.1-2.109
Barium Hydroxide
Introduction
Barium Hydroxide is made up of the elements Ba,H and O. It seems to be a soft, shiny, alkaline earth metal found within Group 2 of the current periodic table. This is scientifically quite reactive. As a result, it would never be discovered in the environment as a pure element. These derivatives are utilised in the petroleum industry as well as radiography, but it is also useful in metallurgy. It is indeed a transparent white granule having no naturally toxic odour. Because of the hydroxides, is harmful or could harm the skin as well as the eyes. Inhaling barium hydroxide across an extended period might induce breathing difficulties. As a result, caution is necessary while using barium hydroxide. It has been a compound composed of barium oxide as well as water. The element Barium's monohydrate, commonly called baryta, is among the most important compounds. It was also named barium di hydroxide as well as corrosive baryta.
Definition of Barium Hydroxide
Barium hydroxide has to be an organic compound that is sometimes also known as baryta as well as baryta-water. It has the structural formula . Its structural properties have included the fact that it appears in industrial form as transparent white granules without odour. This is also regarded as toxic. The whole compound's composition is ionic, and it offers 2 hydroxide ions per molecule in aq. phase. This is a reagent used during the metallization of carboxamides. This even degrades less quickly than barium oxide.
Chemical Structure of Barium Hydroxide
The chemical formula for barium hydroxide is . Thus, this should simply hold 1 barium as well as 1 hydroxide molecule, but to keep the compound balanced, we must add one more hydroxide molecule since barium, as well as hydroxide, have such an uneven amount of ions.
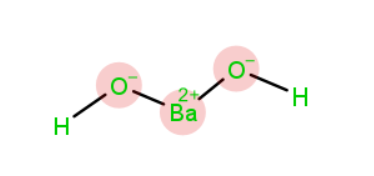
Properties of Barium Hydroxide
Physical Properties
It seems to be a colourless, tasteless, and toxic substance.
It has a molar mass of 171.34g/mol.
The melting point of varies with the amount of water available.
The octahydrate stage melts around 78 C. The monohydrate stage melts around 300C, whereas the anhydrous stage melts at 407.
At relatively low temp, all 3 kinds are partly water-soluble. Therefore, the solubility of water will increase when the temp rises.
The density of is 3.743g/mL (monohydrate) as well as 2.18g/mL (in octahydrate form).
At temperatures exceeding 800 C, boils at 780C as well as breaks down forming .
It seems to be a colourless, tasteless, and toxic substance.
It has a molar mass of 171.34g/mol.
The melting point of varies with the amount of water available.
The octahydrate stage melts around 78 C. The monohydrate stage melts around 300C, whereas the anhydrous stage melts at 407.
At relatively low temp, all 3 kinds are partly water-soluble. Therefore, the solubility of water will increase when the temp rises.
The density of is 3.743g/mL (monohydrate) as well as 2.18g/mL (in octahydrate form).
At temperatures exceeding 800 C, boils at 780C as well as breaks down forming .
Chemical Properties
can provide 2 hydroxide ions per molecule in an aqueous medium, thus it is ionic.
Whether it had been hydrated, which could have 1 to 8 water molecules en\circling the cations as well as anions.
When barium hydroxide dissolves, it generates alkaline solutions. These are caused by the emission of hydroxide anions. As a result, it performs acid neutralisation reactions.
It reacts with sulphuric as well as phosphoric acids and generates valuable Ba salts including as well as .
reacts with to generate , however, when reacts with to yield .
Since aqueous solution is treated with some other metal compounds, double replacement reactions take place, producing several insoluble or even less soluble barium salts.
As interacts with ammonium salts, an endothermic reaction occurs.
can provide 2 hydroxide ions per molecule in an aqueous medium, thus it is ionic.
Whether it had been hydrated, which could have 1 to 8 water molecules en\circling the cations as well as anions.
When barium hydroxide dissolves, it generates alkaline solutions. These are caused by the emission of hydroxide anions. As a result, it performs acid neutralisation reactions.
It reacts with sulphuric as well as phosphoric acids and generates valuable Ba salts including as well as .
reacts with to generate , however, when reacts with to yield .
Since aqueous solution is treated with some other metal compounds, double replacement reactions take place, producing several insoluble or even less soluble barium salts.
As interacts with ammonium salts, an endothermic reaction occurs.
Endothermic Reaction of Barium Hydroxide
Endothermic reactions consume energy from their environment, while exothermic reactions emit energy. For such an endothermic reaction to occur, the reactants should contain more energy than the products. The reaction is accelerated by such an energy gap. For instance, if we combine Barium hydroxide with ammonium chloride. When you combine the 2 chemicals inside a beaker, the temp drops far too rapidly. Whenever you examine the temp within the beaker, you might notice that it would have dropped under - 20 C . The process consumes the ambient heat but also produces ammonia, barium chloride, as well as water.
Uses
It is being used to make various barium salts including , as well as .
It is also useful in analytical methods for weak acid and organic acid calibration. However, except for and , their transparent aqueous solution must be carbonate-free, as is not soluble inside the water.
It has been used to develop a strong corrosive base within aqueous solutions as well as to evaluate sulphides in pesticides.
Because of its high sevoflurane content, initial absorbent usage, as well as high absorbent temp, lime is preferred rather than (soda lime).
Glass, alkali metals, oil as well as grease modifiers, but also for various compounds, and even soaps are also made from it.
It is being used to make various barium salts including , as well as .
It is also useful in analytical methods for weak acid and organic acid calibration. However, except for and , their transparent aqueous solution must be carbonate-free, as is not soluble inside the water.
It has been used to develop a strong corrosive base within aqueous solutions as well as to evaluate sulphides in pesticides.
Because of its high sevoflurane content, initial absorbent usage, as well as high absorbent temp, lime is preferred rather than (soda lime).
Glass, alkali metals, oil as well as grease modifiers, but also for various compounds, and even soaps are also made from it.
Conclusion
Several of the major compounds of is barium hydroxide. It serves as a starting point for the production of numerous barium salts. is a powdery white chemical. This is mainly composed of as well as ‚. The monohydrate recognized as baryta as well as baryta-water is among the most important compounds of the element barium. It is completely dispersed in water as well as consists of barium plus hydroxyl ions. It undergoes a significant endothermic reaction when it reacts with . It is often used to detoxify sewage water, in the laboratories to manufacture various barium compounds. Ions of barium seem to be poisonous to muscles but also can induce paralysis. This is an inorganic compound utilised in chemistry as a source of barium metallic as well as a reagent.
FAQs
Qns. 1. How much charge does have?
Ans. Barium is indeed an atom in Group 2 of the Standard Periodic Table, which means it does have 2 valence electrons as well as a charge of +2. Hydroxide is a negatively charged polyatomic ion including a value of 1. When they combine, they produce an ionic compound having zero charge.
Qns. 2. How often is barium hydroxide synthesised?
Ans. Disintegrating barium oxide in water yields barium hydroxide. It is clear that such a chemical crystallises as octahydrate but has been changed to monohydrate through heating in the air.
Qns. 3. What occurs when you electrolyze a heated aqueous solution?
Ans. The purest source of hydrogen gas is often generated by electrolysis of such a heated solution of the hydroxyl molecule of barium hydroxide. This might be accomplished using both platinum as well as nickel electrodes.
Qns. 4. What effects may barium hydroxide have?
Ans. It damages the respiratory system, eyes, as well as skin. Once obtained via the skin, it is hazardous. Serious harm is also done to the heart as well as the central nervous system.
Qns. 5. Is barium hydroxide considered a salt?
Ans. Whenever barium hydroxide is dispersed in water, it produces alkali solutions resulting in the formation of hydroxyl anions. Although may interact with sulphuric, phosphoric, as well as other acids to generate the corresponding salts, it's being utilised to make barium salts.
Barium Nitrate
Introduction
A salt made of ions of Barium as well as Nitrate is known as Barium Nitrate. It exists naturally as the incredibly uncommon mineral nitrobarite. As an oxidant, barium nitrate interacts violently with common reducing compounds. 2 parts of nitrate & 1 part of barium are combined to create the chemical known as barium nitrate. Barium nitrate's molecular formula is thus represented as Ba(NO3)2.
A salt made of ions of Barium as well as Nitrate is known as Barium Nitrate. It exists naturally as the incredibly uncommon mineral nitrobarite. As an oxidant, barium nitrate interacts violently with common reducing compounds. 2 parts of nitrate & 1 part of barium are combined to create the chemical known as barium nitrate. Barium nitrate's molecular formula is thus represented as Ba(NO3)2.
Advanced Chemistry: What is Barium Nitrate?
An inorganic chemical called barium nitrate is sometimes referred to as barium dinitrate, Nitrobarite, or even barium salt. Barium salt is typically found as a white, crystalline substance. The substance is inherently incombustible. However, it burns with a green flame when used as an oxidizer and can explode if exposed to flame for a prolonged period.
Structure
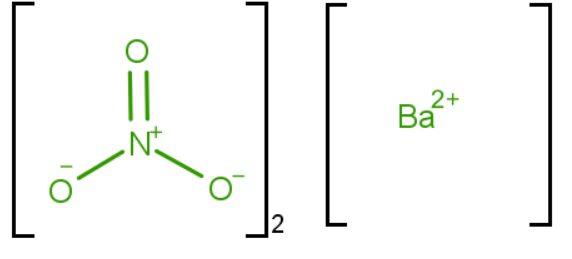
One Barium cation Ba2+ and 2 nitrate anions NO−3 to form. Moreover, the crystal structure is cubic with 1 barium cation surrounded by 4 nitrate anions.
An inorganic chemical called barium nitrate is sometimes referred to as barium dinitrate, Nitrobarite, or even barium salt. Barium salt is typically found as a white, crystalline substance. The substance is inherently incombustible. However, it burns with a green flame when used as an oxidizer and can explode if exposed to flame for a prolonged period.
Structure

One Barium cation Ba2+ and 2 nitrate anions NO−3 to form. Moreover, the crystal structure is cubic with 1 barium cation surrounded by 4 nitrate anions.
Properties of Barium Nitrate
Physical properties
Properties Barium Nitrate Molecular Weight 261.37 g/mol Appearance Crystalline solid Colour White Combustibility Non-combustible Melting Point 590 ∘C Odour Odourless Boiling point 83 ∘C IUPAC Name Barium(2+) dinitrate Refractive Index 1.5659
Images Coming soon
| Properties | Barium Nitrate |
|---|---|
| Molecular Weight | 261.37 g/mol |
| Appearance | Crystalline solid |
| Colour | White |
| Combustibility | Non-combustible |
| Melting Point | 590 ∘C |
| Odour | Odourless |
| Boiling point | 83 ∘C |
| IUPAC Name | Barium(2+) dinitrate |
| Refractive Index | 1.5659 |
Images Coming soon
Chemical properties
It is a very powerful oxidising agent that reacts violently with metals. Barium nitrate is frequently used for military purposes since it contains a significant amount of nitrate. For instance, it is used to create explosives such as grenades. Despite not being combustible itself, it improves the combustibility of other compounds. Barium nitrate should not be exposed to intense heat for an extended period since it may explode or provide a fire danger. Additionally, it serves as an oxidising agent for many chemical processes; this ability has a range of uses.
It may split down or decompose at high temperatures to produce Barium Oxide and Nitrogen Dioxide.
2Ba(NO3)2→2BaO+4NO2+O2
It is a very powerful oxidising agent that reacts violently with metals. Barium nitrate is frequently used for military purposes since it contains a significant amount of nitrate. For instance, it is used to create explosives such as grenades. Despite not being combustible itself, it improves the combustibility of other compounds. Barium nitrate should not be exposed to intense heat for an extended period since it may explode or provide a fire danger. Additionally, it serves as an oxidising agent for many chemical processes; this ability has a range of uses.
It may split down or decompose at high temperatures to produce Barium Oxide and Nitrogen Dioxide.
2Ba(NO3)2→2BaO+4NO2+O2
How to Produce Barium Nitrate
Method 1
Barium carbonate is initially dissolved in HNO3. Iron impurities precipitate to the surface as these chemicals react. You must eliminate these contaminants using the filtration procedure. The leftover mixture must then be evaporated after that. After crystallisation, we finally obtain pure Ba(NO3)2, which is frequently used in industry.
BaCO3+2HNO3→Ba(NO3)2+CO2+H2O
Barium carbonate is initially dissolved in HNO3. Iron impurities precipitate to the surface as these chemicals react. You must eliminate these contaminants using the filtration procedure. The leftover mixture must then be evaporated after that. After crystallisation, we finally obtain pure Ba(NO3)2, which is frequently used in industry.
BaCO3+2HNO3→Ba(NO3)2+CO2+H2O
Method 2
The first step in the preparation of barium nitrate is either the reaction of HNO3 and Ba or HNO3 and BaO.
2HNO3+Ba→Ba(NO3)2+H2
2HNO3+BaO→Ba(NO3)2+H2O
The first step in the preparation of barium nitrate is either the reaction of HNO3 and Ba or HNO3 and BaO.
2HNO3+Ba→Ba(NO3)2+H2
2HNO3+BaO→Ba(NO3)2+H2O
Precautionary Measures to Follow While Handling Barium Nitrate
This substance can be severely harmful to humans in high doses. Be careful not to breathe in too many of its fumes. Several hazardous symptoms can also result from direct exposure to the skin & eyes. Some symptoms of barium nitrate poisoning include upper lung inflammation, skin irritation, & eye irritation. Notably, they are easily avoidable by using basic safety precautions when handling them.
This substance can be severely harmful to humans in high doses. Be careful not to breathe in too many of its fumes. Several hazardous symptoms can also result from direct exposure to the skin & eyes. Some symptoms of barium nitrate poisoning include upper lung inflammation, skin irritation, & eye irritation. Notably, they are easily avoidable by using basic safety precautions when handling them.
Applications of Barium Nitrate
Some of these major uses are discussed below in detail.
The home's wall paints are just a combination of different chemical compounds. One of these substances is barium nitrate. Notably, because it is frequently utilised as a fundamental component, the majority of paintings would not exist without this specific barium nitrate.
A primary element in the production of premium glass products is barium nitrate. The oxygen molecule that is connected to this substance can aid in preventing the discolouration of glass objects. Such glassware is also incredibly transparent. When making optical glasses, experts also employ this substance. Clearer images can be obtained by using barium nitrate in camera lenses to increase the refractive index.
Because it can aid in the creation of green flames, barium nitrate is a prized substance in the fireworks industry. One can detect the usage of this substance in a variety of pyrotechnic displays, in addition to fireworks. Similar to how barium nitrate produces green flames, sodium chloride produces yellow flames, while copper chloride burns with a blue flame. As a result, fireworks frequently combine all of these.
Making tracer bullets is aided by barium nitrate. Thanks to a Ba(NO3)2, charge put directly at the end of such ammunition, 1 can precisely trace the path the bullet follows when shot. When such bullets are shot from a gun, this charge ignites. This area's smoke can be seen, clearly showing the bullet's route.
Barium nitrate is also one of the main ingredients used to make flares, which are used to direct troops to a specific spot. Finally, this substance is also present in explosive detonators. Likewise, barium nitrate is a crucial component of explosives because of its oxidising nature.
Barium nitrate works well as a rodenticide as well. It will keep different rodents, such as mice & rats, from entering or remaining within the premises if you leave it outside your home.
Ba(NO3)2 can occasionally be used to make specific kinds of propellants. However, pyrotechnic and military applications continue to be their primary uses.
Some of these major uses are discussed below in detail.
The home's wall paints are just a combination of different chemical compounds. One of these substances is barium nitrate. Notably, because it is frequently utilised as a fundamental component, the majority of paintings would not exist without this specific barium nitrate.
A primary element in the production of premium glass products is barium nitrate. The oxygen molecule that is connected to this substance can aid in preventing the discolouration of glass objects. Such glassware is also incredibly transparent. When making optical glasses, experts also employ this substance. Clearer images can be obtained by using barium nitrate in camera lenses to increase the refractive index.
Because it can aid in the creation of green flames, barium nitrate is a prized substance in the fireworks industry. One can detect the usage of this substance in a variety of pyrotechnic displays, in addition to fireworks. Similar to how barium nitrate produces green flames, sodium chloride produces yellow flames, while copper chloride burns with a blue flame. As a result, fireworks frequently combine all of these.
Making tracer bullets is aided by barium nitrate. Thanks to a Ba(NO3)2, charge put directly at the end of such ammunition, 1 can precisely trace the path the bullet follows when shot. When such bullets are shot from a gun, this charge ignites. This area's smoke can be seen, clearly showing the bullet's route.
Barium nitrate is also one of the main ingredients used to make flares, which are used to direct troops to a specific spot. Finally, this substance is also present in explosive detonators. Likewise, barium nitrate is a crucial component of explosives because of its oxidising nature.
Barium nitrate works well as a rodenticide as well. It will keep different rodents, such as mice & rats, from entering or remaining within the premises if you leave it outside your home.
Ba(NO3)2 can occasionally be used to make specific kinds of propellants. However, pyrotechnic and military applications continue to be their primary uses.
Conclusion
It can be concluded that barium nitrate is crystalline. It is a salt with a very slight acidic flavour. Despite being non-flammable in most situations, this chemical can explode at very high temperatures. As an oxidising agent, barium nitrate promotes the burning of other substances. There are two ways to make this chemical. This substance has a melting point of around 590 ∘ C. It simply decomposes at high temperatures. People who are exposed to this material may experience barium nitrate poisoning symptoms, such as well as skin irritation.
It can be concluded that barium nitrate is crystalline. It is a salt with a very slight acidic flavour. Despite being non-flammable in most situations, this chemical can explode at very high temperatures. As an oxidising agent, barium nitrate promotes the burning of other substances. There are two ways to make this chemical. This substance has a melting point of around 590 ∘ C. It simply decomposes at high temperatures. People who are exposed to this material may experience barium nitrate poisoning symptoms, such as well as skin irritation.
FAQs
Q1. Is barium nitrate salt insoluble in nature?
Ans. All nitrate salts are soluble. Because it can be absorbed by the body, barium nitrate's solubility makes it very dangerous to people.
Q 2. Barium nitrate burns green and why?
Ans. When heated to a high temperature, several substances emit a distinctive colour. The metal ions are excited by the flame's heat, which makes them radiate visible light. Barium emits light at a wavelength that corresponds to the colour green, which is why it has a green hue.
Q3. What product will be formed when we burn barium nitrate?
Ans. Barium nitrate burns with a green flame. When heated, it breaks down into barium oxide, oxygen, & nitrogen dioxide. As a result, nitric dioxide is produced from barium nitrate.
2Ba(NO3)2→2BaO+4NO2+O2
Q4. Is it possible to make Barium Sulphate soluble in water?
Ans. The chemical will be soluble in water if the hydration energy of the ions is high enough to outweigh the molecule's lattice energy. It won't dissolve in water if the hydration energy of the ions does not exceed the lattice energy of the molecule. In the case of Barium Sulphate, the size of Barium ions and sulphate ions is almost similar. Because of this, the lattice energy of the molecule will get higher and which will overcome the hydration energy. Hence, it is not possible to make Barium sulphate soluble in water.
Qns. 5. Why Barium Nitrate does not react with Sulphuric acid?
Ans. Reacting Barium Nitrate with Sulphuric acid will produce Barium Sulphate and Nitric acid. As Barium sulphate is insoluble in water it will form a white precipitate out of the solution.
Ba(NO3)2+H2SO4→BaSO4+2HNO3
Q1. Is barium nitrate salt insoluble in nature?
Ans. All nitrate salts are soluble. Because it can be absorbed by the body, barium nitrate's solubility makes it very dangerous to people.
Q 2. Barium nitrate burns green and why?
Ans. When heated to a high temperature, several substances emit a distinctive colour. The metal ions are excited by the flame's heat, which makes them radiate visible light. Barium emits light at a wavelength that corresponds to the colour green, which is why it has a green hue.
Q3. What product will be formed when we burn barium nitrate?
Ans. Barium nitrate burns with a green flame. When heated, it breaks down into barium oxide, oxygen, & nitrogen dioxide. As a result, nitric dioxide is produced from barium nitrate.
2Ba(NO3)2→2BaO+4NO2+O2
Q4. Is it possible to make Barium Sulphate soluble in water?
Ans. The chemical will be soluble in water if the hydration energy of the ions is high enough to outweigh the molecule's lattice energy. It won't dissolve in water if the hydration energy of the ions does not exceed the lattice energy of the molecule. In the case of Barium Sulphate, the size of Barium ions and sulphate ions is almost similar. Because of this, the lattice energy of the molecule will get higher and which will overcome the hydration energy. Hence, it is not possible to make Barium sulphate soluble in water.
Qns. 5. Why Barium Nitrate does not react with Sulphuric acid?
Ans. Reacting Barium Nitrate with Sulphuric acid will produce Barium Sulphate and Nitric acid. As Barium sulphate is insoluble in water it will form a white precipitate out of the solution.
Ba(NO3)2+H2SO4→BaSO4+2HNO3
Barium Oxide
Introduction
Because barium does not exist naturally as a free component, it is obtained in ores, usually in the state of barite (BaSO4). Barium constitutes 0.05 per cent of the earth's crust. It is an alkaline earth metal with a light yellow as well as a gleaming look. Carl Wilhelm Scheele (C.W.S.) discovered barium in the year 1772 when he demonstrated that BaO was separate from CaO‚ a substance it was frequently confused for. Humphry Davy separated Ba for the 1st time in the year 1808 using an electrolysis process he created.
Barium oxide BaO, commonly known as barium monoxide, oxobarium, or baria is a non-flammable yellow-white substance. And it has a chemical formula BaO which is hygroscopic, meaning it quickly collects moisture from the air. Barium (Ba), in different compounds, has a wide range of uses, including use in the oil drilling, paint industry, glassware, ceramic manufacture as well as, drying agent in medicine, fireworks, including electronics among others.
Because barium does not exist naturally as a free component, it is obtained in ores, usually in the state of barite (BaSO4). Barium constitutes 0.05 per cent of the earth's crust. It is an alkaline earth metal with a light yellow as well as a gleaming look. Carl Wilhelm Scheele (C.W.S.) discovered barium in the year 1772 when he demonstrated that BaO was separate from CaO‚ a substance it was frequently confused for. Humphry Davy separated Ba for the 1st time in the year 1808 using an electrolysis process he created.
Barium oxide BaO, commonly known as barium monoxide, oxobarium, or baria is a non-flammable yellow-white substance. And it has a chemical formula BaO which is hygroscopic, meaning it quickly collects moisture from the air. Barium (Ba), in different compounds, has a wide range of uses, including use in the oil drilling, paint industry, glassware, ceramic manufacture as well as, drying agent in medicine, fireworks, including electronics among others.
What is barium oxide?
Thermally stable barium oxide BaO is very insoluble. When BaO reacts with water, it generates barium hydroxide Ba(OH2). It is a white hygroscopic material that is typically generated through the breakdown of barium salts. Oxide complexes are not electrically conductive. Some perovskite structured oxides, on the other hand, are electrically conductive but have found use in the cathode of solid oxide fuel cells for oxygen production systems. They are substances that include at least 1 oxygen anion as well as 1 metallic cation. They are often insoluble in aqueous solutions and also exceedingly durable, making them suitable in ceramic structures ranging from basic clay bowls to complex electronics with lightweight structural parts in aerospace for electrochemical purposes such as fuel cells. Because metal oxide complexes are basic anhydrides, they can undergo redox reactions with acids as well as act as a strong reducing agent. Pellets, bits, sputtering targets, powder, tablets, and even nanopowder are also available. Most quantities of BaOare readily obtainable. Powder forms such as ultrahigh purity, high purity, submicron, but also nano may be explored.
Thermally stable barium oxide BaO is very insoluble. When BaO reacts with water, it generates barium hydroxide Ba(OH2). It is a white hygroscopic material that is typically generated through the breakdown of barium salts. Oxide complexes are not electrically conductive. Some perovskite structured oxides, on the other hand, are electrically conductive but have found use in the cathode of solid oxide fuel cells for oxygen production systems. They are substances that include at least 1 oxygen anion as well as 1 metallic cation. They are often insoluble in aqueous solutions and also exceedingly durable, making them suitable in ceramic structures ranging from basic clay bowls to complex electronics with lightweight structural parts in aerospace for electrochemical purposes such as fuel cells. Because metal oxide complexes are basic anhydrides, they can undergo redox reactions with acids as well as act as a strong reducing agent. Pellets, bits, sputtering targets, powder, tablets, and even nanopowder are also available. Most quantities of BaOare readily obtainable. Powder forms such as ultrahigh purity, high purity, submicron, but also nano may be explored.
What is the formula for barium oxide?
It is produced by oxidising Ba salts such as barium carbonate (BaCO3), in which the barium (Ba) burns in oxygen (O) to generate BaO. As a result, Barium Oxide has the chemical formula BaO.
2Ba+O2→2Ba+2BaO
It is produced by oxidising Ba salts such as barium carbonate (BaCO3), in which the barium (Ba) burns in oxygen (O) to generate BaO. As a result, Barium Oxide has the chemical formula BaO.
2Ba+O2→2Ba+2BaO
Structure of barium oxide
It has a cubic structure, the barium oxide structure (BaO), in which 1 ion of barium (Ba) participates in the process but both ions combine to produce BaO. As a result, barium (Ba) is oxidized to generate barium oxide BaO.
It has a net monoisotopic mass of 153.9 g/mol. Because the no. of hydrogen (H) bond acceptors is 1 and the no. of hydrogen (H) bond donors is 0, the chemical contains 1 covalently bonded unit.
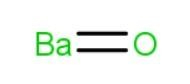
It has a cubic structure, the barium oxide structure (BaO), in which 1 ion of barium (Ba) participates in the process but both ions combine to produce BaO. As a result, barium (Ba) is oxidized to generate barium oxide BaO.
It has a net monoisotopic mass of 153.9 g/mol. Because the no. of hydrogen (H) bond acceptors is 1 and the no. of hydrogen (H) bond donors is 0, the chemical contains 1 covalently bonded unit.
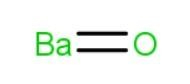
Properties of barium oxide (BaO)
Property Attributes Molecular weight 153.326 g/mol Magnetic susceptibility −29.110−6cm3/mol Density 5.72g/cm3 Solubility in water 3.48 g/100 ml at 20 ∘C B.P. 2000 ∘C Crystal structure Cubic M.P. 1923 ∘C
| Property | Attributes |
|---|---|
| Molecular weight | 153.326 g/mol |
| Magnetic susceptibility | −29.110−6cm3/mol |
| Density | 5.72g/cm3 |
| Solubility in water | 3.48 g/100 ml at 20 ∘C |
| B.P. | 2000 ∘C |
| Crystal structure | Cubic |
| M.P. | 1923 ∘C |
Production of barium oxide
Thermal decomposition of Barium Carbonate BaCO3, or Barium Nitrate Ba(NO3)2, can provide Barium OxideBaO. The following are the reactions
BaCO3→BaO+CO2
2Ba(NO3)2→2BaO+4NO2+O2
It can also be made by combining Ba with O.
2Ba+O2→2BaO
It has also been produced by reacting barium chloride (BaCl2), with ammonia (NH3), then precipitating it with deionized water. This is one of the numerous procedures for manufacturing (BaO) that can only be done in a lab under controlled circumstances.
Industrially, the extraction of barium oxide (BaO) from barium carbonate (BaCl2) faces extraction challenges. This is because it is frequently polluted with extra carbon, which is normally supplied to avoid BaO from converting to barium peroxide. The existence of extra carbon causes the combination of carbon (C) including barium oxide BaO to look black. It is not possible to remove the extra carbon (C) merely by warming the mixture since this would trigger a reaction with the (BaO) for regeneration of barium carbonate (BaCO3), thus undoing the progress accomplished.
Thermal decomposition of Barium Carbonate BaCO3, or Barium Nitrate Ba(NO3)2, can provide Barium OxideBaO. The following are the reactions
BaCO3→BaO+CO2
2Ba(NO3)2→2BaO+4NO2+O2
It can also be made by combining Ba with O.
2Ba+O2→2BaO
It has also been produced by reacting barium chloride (BaCl2), with ammonia (NH3), then precipitating it with deionized water. This is one of the numerous procedures for manufacturing (BaO) that can only be done in a lab under controlled circumstances.
Industrially, the extraction of barium oxide (BaO) from barium carbonate (BaCl2) faces extraction challenges. This is because it is frequently polluted with extra carbon, which is normally supplied to avoid BaO from converting to barium peroxide. The existence of extra carbon causes the combination of carbon (C) including barium oxide BaO to look black. It is not possible to remove the extra carbon (C) merely by warming the mixture since this would trigger a reaction with the (BaO) for regeneration of barium carbonate (BaCO3), thus undoing the progress accomplished.
Uses of barium oxide
In electrical equipment, to cover hot cathodes
In the process between alcohols with ethylene oxide, as an ethoxylation catalyst
Through thermal fluctuation, as an oxygen (O) source
For oxidation of barium peroxide
In isomer separation procedures
As a reducing agent
As an oxidizing agent
To boost the flux density of permanent magnets
Lubricating oil detergents are used as a cleaning agent in fuels.
Used as a desiccant to dry gases including liquids. It has the added benefit of not swelling with moisture but also getting wet plus sticky.
In electrical equipment, to cover hot cathodes
In the process between alcohols with ethylene oxide, as an ethoxylation catalyst
Through thermal fluctuation, as an oxygen (O) source
For oxidation of barium peroxide
In isomer separation procedures
As a reducing agent
As an oxidizing agent
To boost the flux density of permanent magnets
Lubricating oil detergents are used as a cleaning agent in fuels.
Used as a desiccant to dry gases including liquids. It has the added benefit of not swelling with moisture but also getting wet plus sticky.
Conclusion
At ambient temperature,BaO is a white, one-of-a-kind chemical substance that can absorb water from its surroundings. It is generated when Ba salts, such as BaCO3, are heated. It is also utilized as a liquid drying agent. Several Ba-containing oxides also include other metals. They are frequently tailored for specific uses but are usually in powdered form with particle sizes as tiny as thirty nanometers.
It is a poisonous as well as a corrosive substance that is non-combustible but also sensitive to moisture. Inhaling, ingesting, or coming into contact with vapours, substances, or dust results in serious burns or deaths.
At ambient temperature,BaO is a white, one-of-a-kind chemical substance that can absorb water from its surroundings. It is generated when Ba salts, such as BaCO3, are heated. It is also utilized as a liquid drying agent. Several Ba-containing oxides also include other metals. They are frequently tailored for specific uses but are usually in powdered form with particle sizes as tiny as thirty nanometers.
It is a poisonous as well as a corrosive substance that is non-combustible but also sensitive to moisture. Inhaling, ingesting, or coming into contact with vapours, substances, or dust results in serious burns or deaths.
FAQs
Q1. What happens when you consume barium monoxide?
Ans. Excessive amounts of barium oxide can cause death. Barium protoxide has a cubic structure that seems like a non-flammable white-yellow powder. When consumed, it may be harmful. Irritating to the eyes, mucous membranes, as well as skin. BaO has an accurate mass as well as a monoisotopic mass of 153.9 g/mol.
Q2. What chemical characteristics does BaO have?
Ans. When a barium salt is oxidized, it produces barium monoxide, also known as baria. It is a white hygroscopic material that is non-combustible.
Due to the oxidation of barium salts, the sub-atomic expression for barium oxide is BaO. The expression for barium oxide is given by the accompanying condition,
2Ba+O2→2BaO
Q3. In sodium (Na) vapour lamps, why do we treat tungsten (W) cathodes with barium oxide(BaO)?
Ans. When BaO is accumulated on the wire filament, the working ability of W is reduced. To put it differently, tungsten (W) discharges electrons more effectively and thus functions more effectively.
Q4. How is carbon introduced to the reaction mixture during the thermal breakdown of BaCO3 to produce BaO?
Ans. Due to its high lattice energy, barium carbonate decomposes at around 1400 ∘C.
BaO+CO2→BaCO3
The process becomes reversible at such high temperatures. Carbon added reacts with CO2 to produce CO, making the process irreversible,CO2+C→2CO.
Q5. What exactly is a Barium (Ba) enema?
Ans. It is an X-ray diagnostic that can detect abnormalities or anomalies in the digestive system (colon). The system is also known as a colon X-ray. A purification is when fluid is infused into your rectum via a tiny tube. In this case, the fluid contains a metallic component (barium) that coats the colon's covering. Normally, an X-ray produces an unfavourable image of sensitive tissues, but the Ba coating produces a usually clear image of the colon.
Q1. What happens when you consume barium monoxide?
Ans. Excessive amounts of barium oxide can cause death. Barium protoxide has a cubic structure that seems like a non-flammable white-yellow powder. When consumed, it may be harmful. Irritating to the eyes, mucous membranes, as well as skin. BaO has an accurate mass as well as a monoisotopic mass of 153.9 g/mol.
Q2. What chemical characteristics does BaO have?
Ans. When a barium salt is oxidized, it produces barium monoxide, also known as baria. It is a white hygroscopic material that is non-combustible.
Due to the oxidation of barium salts, the sub-atomic expression for barium oxide is BaO. The expression for barium oxide is given by the accompanying condition,
2Ba+O2→2BaO
Q3. In sodium (Na) vapour lamps, why do we treat tungsten (W) cathodes with barium oxide(BaO)?
Ans. When BaO is accumulated on the wire filament, the working ability of W is reduced. To put it differently, tungsten (W) discharges electrons more effectively and thus functions more effectively.
Q4. How is carbon introduced to the reaction mixture during the thermal breakdown of BaCO3 to produce BaO?
Ans. Due to its high lattice energy, barium carbonate decomposes at around 1400 ∘C.
BaO+CO2→BaCO3
The process becomes reversible at such high temperatures. Carbon added reacts with CO2 to produce CO, making the process irreversible,CO2+C→2CO.
Q5. What exactly is a Barium (Ba) enema?
Ans. It is an X-ray diagnostic that can detect abnormalities or anomalies in the digestive system (colon). The system is also known as a colon X-ray. A purification is when fluid is infused into your rectum via a tiny tube. In this case, the fluid contains a metallic component (barium) that coats the colon's covering. Normally, an X-ray produces an unfavourable image of sensitive tissues, but the Ba coating produces a usually clear image of the colon.

No comments:
Post a Comment