Aldol condensation and Crossed aldol condensation reaction- Definition, Examples, Mechanism and Uses.
Contents [hide]
- Aldol condensation reaction
- Mechanism of aldol condensation reaction
- Dehydration of aldol products
- Uses of aldol condensation in synthesis
- Crossed aldol condensation reaction
- Mechanism of crossed-aldol condensation reaction
- References
Aldol condensation reaction
Aldol condensation reaction is one of the important reactions of carbonyl compounds (i.e. aldehydes and ketones)
Condensation between two molecules of aldehydes or ketones having at least one α – hydrogen atom in presence of dilute alkali to form β-hydroxy aldehyde or β-hydroxy ketone is known as aldol condensation reaction. Examples:
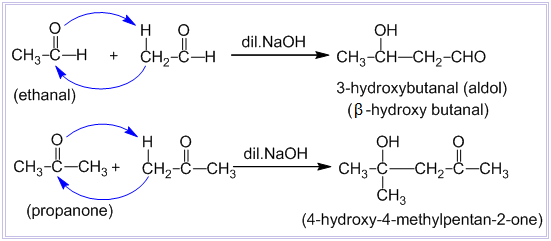
Aldehydes and ketones which do not contain any α – hydrogen atom such as HCHO, (CH3)3CCHO, C6H5CHO, etc. do not undergo aldol condensation reaction.
Mechanism of aldol condensation reaction
Taking acetaldehyde (ethanal) as an example, aldol condensation involves following steps:
Step-I :
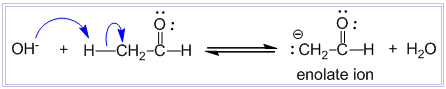
In this step, hydroxide ion from alkali removes a proton from the α – carbon of one molecule of ethanal to give a carbanion (i.e. enolate ion).
Step-II :
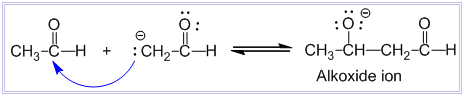
In this step, there is nucleophilic addition of enolate ion to the carbonyl carbon of second molecule of ethanal to produce an alkoxide ion.
Step-III :
In this step, the alkoxide ion takes up a proton from water to form β-hydroxy aldehyde (aldol).

Dehydration of aldol products
The product of aldol condensation on heating with dilute acids undergo dehydration to form α, β-unsaturated aldehydes or ketones. Examples:

Uses of aldol condensation in synthesis
Aldol products contain two functional groups (-OH and –CHO) and hence they can be used in a number of reactions to give various products. For example:
1. Catalytic hydrogenation of α, β-unsaturated aldehydes or ketones, obtained from dehydration of aldol products gives saturated alcohols.

2. Reduction of α, β-unsaturated aldehydes or ketones by LiAlH4 gives unsaturated alcohol.

Crossed aldol condensation reaction
- Aldol condensation between two different carbonyl compounds is called a crossed aldol condensation.
- Crossed aldol condensation is not useful in laboratories if both of the carbonyl compounds have α-hydrogen, because a mixture of products are formed.
- However, it is useful when one of the carbonyl compounds does not have α-hydrogen and therefore cannot undergo self condensation. For example: benzaldehyde can be used with other aldehydes and ketones containing α-hydrogen.

Mechanism of crossed-aldol condensation reaction
[same as that of aldol condensation reaction]
Taking acetaldehyde (ethanal) and benzaldehyde as examples of aldehydes, crossed aldol condensation involves following steps:
Step-I :
In this step, hydroxide ion from alkali removes a proton from the α – carbon of ethanal to give a carbanion (i.e. enolate ion).

Step-II :
In this step, there is nucleophilic addition of enolate ion to the carbonyl carbon of benzaldehyde to produce an alkoxide ion.
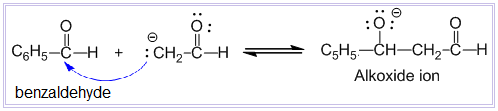
Step-III :
In this step, the alkoxide ion takes up a proton from water to form β-hydroxy aldehyde (aldol).

References
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Morrison, R.T. , Boyd, R.N., Organic Chemistry, Sixth edition, Prentice-Hall of India Pvt. Ltd., 2008.
- March, j., Advanced Organic Chemistry, Fourth edition, Wiley Eastern Ltd. India, 2005.