Chapter 1 : The Solid State
Solid: Solid is a state of matter in which the constituting particles are arranged very closely. The constituent particles can be atoms, molecules or ions.
Properties of solids:
- They have definite mass, volume and shape.
- They are incompressible and rigid.
- Intermolecular distances are very short and hence the intermolecular forces are strong.
- Their constituent particles have fixed positions and can only oscillate about their mean positions.
- They have very low rates of diffusion.
- They can exist in crystalline state.
- They have high density.
- They exhibit sharp melting point (m .p.).
- They show properties like malleability, ductility, elasticity, brittleness.
- Some of them are good conductors of heat and electricity.
Classification of on the basis of the arrangement of constituent particles:
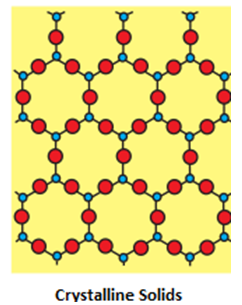
- Properties of crystalline solids:
- Crystalline Geometry : They have a definite geometrical shape.
- Atomic Arrangements: They have a long range order.
- Melting points : They have a sharp melting point.
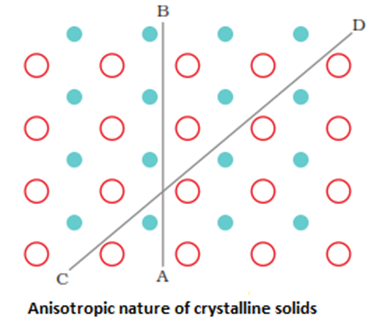
- Isotropy and Anisotropy: They are anisotropic in nature i.e. their physical properties show different values when measured along different directions in the same crystal.
- Cleavages : When cut with a sharp edged tool , they split into two pieces and the newly generated surfaces are plain and smooth.
- Enthalpy of Fusion : They have a definite and characteristic heat of fusion.
- True or Pseudo : They are called true solids as they sustain their shapes over long ranges of time.
- Polymorphic forms or polymorphs:
The different crystalline forms of a substance are known as polymorphic forms or polymorphs .For example: graphite and diamond.
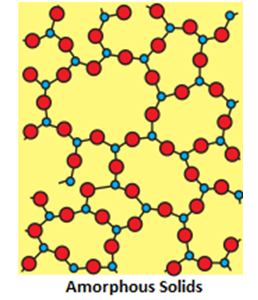
- Characteristics of amorphous solids:
- Crystalline Geometry : They have an irregular shape.
- Atomic Arrangements: They have a short range order.
- Melting points :They gradually soften over arrange of temperature.
- Isotropy and Anisotropy: They are isotropic in nature i.e. their physical properties are the same in all directions.
- Cleavages : When cut with a sharp edged tool, they cut into two pieces with irregular surfaces.
- Enthalpy of Fusion : They do not have definite heat of fusion.
- True or Pseudo: They are called pseudo solids or super cooled liquids. This is because they have a tendency to flow, though very slowly.
- Types of crystalline solids:
A. Molecular Solids
Constituent Particles: Molecules
Bonding/Attractive Forces: London dispersive Forces
Electrical Conductivity: Poor
Physical Nature: Soft and Volatile
Melting Point: Low
Examples: Solid HCl, Solid SO2, Solid H2S, Solid H2, Solid Cl2, Solid Br2, I2, Solid CO2, Water (ice), Solid HF, Solid p- nitrophenol etc.
Properties of Molecular Solids
- They are soft and generally gaseous or liquids at room temperature.
- They are volatile in nature.
- They have low enthalpy of vaporization.
- They have low melting and boiling points.
- They are bad conductor of heat and electricity
B. Ionic Solids
Bonding/Attractive Forces: Coulombic or Electrostatic
Electrical Conductivity: Insulators in solid state but conducts in molten state and in aqueous solutions
Physical Nature: Hard but brittle
Melting Point: High
Examples: , ZnS, MgO, NaCl, KBr,
Properties of Ionic Solids
- They are very hard and brittle
- They have very high melting and boiling point.
- They are non-volatile compounds.
- They are soluble in polar solvent (like H₂O).
- Their ions are not free to move in solid state, so they are insulators of electricity but in molten or aqueous solution their ions free to move so become good conductor of electricity.
C. Metallic Solids
Constituent Particles: Positive ions in a sea of delocalized electrons
Bonding/Attractive Forces: Metallic bonding
Electrical Conductivity: Conductors in solid state as well as in molten state
Physical Nature: Hard but malleable and ductile
Melting Point: Fairly high
Examples: Fe ,Cu, Ag, Mg
- They are soft as well as hard.
- They are good conductor of heat and electricity.
- They have property of malleable and ductile that means can be beaten into sheets and drawn into wires.
- They have high melting and boiling point (except Hg and Cs).
- They have high enthalpy of fusion.
- They are characterized by their ability to reflect light, called metallic luster or metallic shine.
D. Covalent or Network Solids
Constituent Particles: Atoms
Bonding/Attractive Forces: Covalent bonding
Electrical Conductivity: Conductors in solid state as well as in molten state
Physical Nature: Hard but malleable and ductile
Melting Point: Fairly high
Examples: , (quartz), SiC, C (diamond), C(graphite)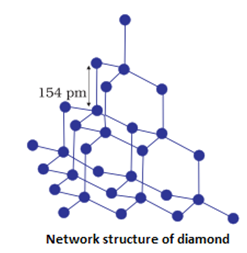
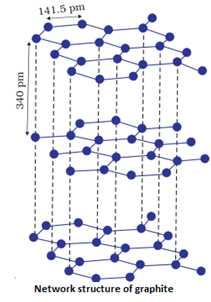
- They are very hard and brittle.
- They have very high melting and boiling point and may even decompose before melting.
- They are insulators of electricity.
- They show property of Polymorphism (Allotropy).
- Crystal lattice: A regular ordered arrangement of constituent particles in three dimensions is called crystal lattice.
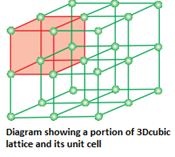
- Lattice points or lattice sites: The fixed positions on which the constituent particles are present are called lattice points or lattice sites. A group of lattice points which when repeated over and over again in three dimensions give the complete crystal lattice.
- Unit cell: It is defined as the smallest repeating unit in space lattice which when repeated over and over again generates the complete crystal lattice. The crystal can consist of an infinite number of unit cells.
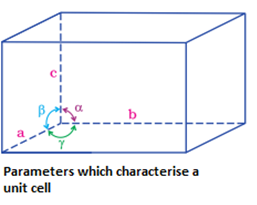
- Parameters which characterize a unit cell:
- Dimensions of the unit cell along the three edges ,a, b and c: These edges may or may not be mutually perpendicular.
- Inclination of the edges to each other: This is denoted by the angle between the edges , , and respectively.
is the angle between the edges b and c.
is the angle between the edges c and a .
is the angle between the edges a and b.
- Seven crystal systems:
- Types of unit cells:
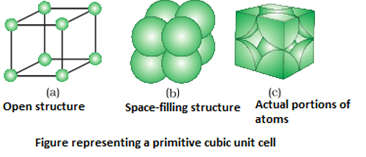
- Primitive or simple unit cells have constituent particles only at its corners.
- Centered unit cells are those unit cells in which one or more constituent particles are present at positions in addition to those present at the corners.
- Types of centered unit cells:
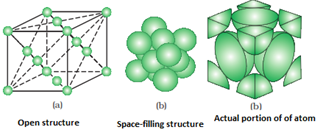
- Face centered unit cell: It consists of one constituent particle present at the centre of each face in addition to those present at the corners.
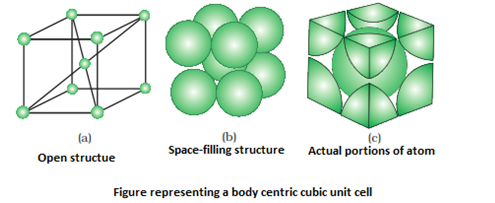
- Body centered unit cell: It consists of a one constituent particle is present at its body centre in addition to those present at the corners.
- End centered unit cell: It consists of one constituent particle present at the centre of any two opposite faces in addition to those present at the corners.
- Number of particles at different lattice positions:
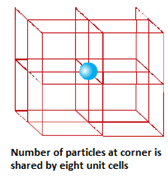
- Face centre: if an atom is present at the centre of the face, it is shared by two unit cells. So, only half of the atom actually belongs to the unit cell.
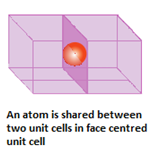
- Body centre: if an atom is present at the body centre, it is not shared by any other unit cell. So that one atom completely belongs to the same unit cell.
- End centre: f an atom is present at the edge centre, it is shared by four unit cells. So, only one fourth of an atom belongs to the unit cell.
- Number of atoms in different unit cells:
- Primitive unit cell have 1atom
- Face centered unit cell have 3 atoms
- Body centered unit cell have 2atoms
- Coordination number: Coordination number is the number of nearest neighbours of a particle.
- Close packed structures:

- Close packing in two dimensions: It is generated by stacking the rows of close packed spheres in two ways:
- i) Square close packing and
- ii) Hexagonal close packing.
- Close packing in three dimensions:
- They can be obtained by stacking the two dimensional layers one above the other. It can be obtained in two ways:
- i) Square close packed layers and
- ii) Hexagonal close packed layers.
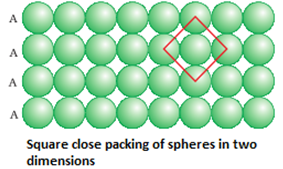
- Square close packing: Here, the spheres of the second row ware placed exactly above those of the first row. This way the spheres are aligned horizontally as well as vertically. The arrangement is AAA type. The coordination number is 4.
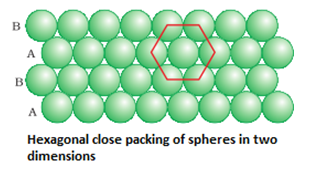
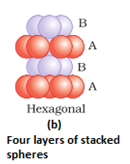
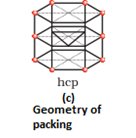
- Hexagonal close packing: Here, these spheres of these bond row are placed above the first one in as staggered manner in such a way that its spheres fit in the depression of the first row. The arrangement is ABAB type. The coordination number is 6.
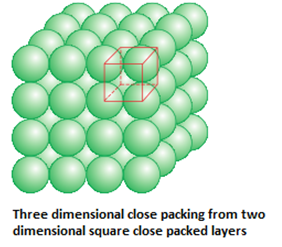
- Three dimensional close packing from two dimensional square close packed layers: Here , the spheres of the upper layer are placed exactly over the first layer such the spheres of the layers are perfectly aligned horizontally and vertically. It has a AAAA type pattern. The lattice is simple cubic lattice.
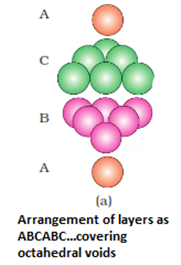
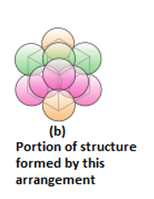
- Three dimensional close packing from two dimensional hexagonal close packed layers: There are two steps involved as:
- Placing these bond layer over the first layer
- Placing the third layer over the second layer
There are two possibilities:
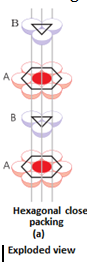
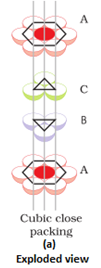
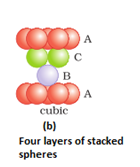
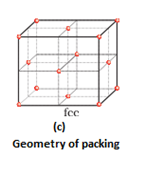
- Covering the octahedral voids: Here, octahedral voids of these bond layer may be covered by the spheres of the third layer. It gives rise to ABCABCABC type pattern. The three dimensional structure is called cubic close packed structure or face centered cubic structure. The coordination number is 12.Example: Cu, Ag.
- Types of voids:
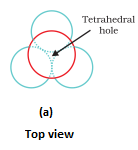
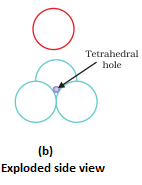
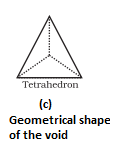
- Octahedral void- It is formed at the centre when six spheres are joined in the form of an octahedron.
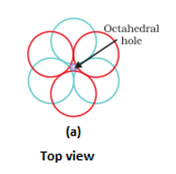
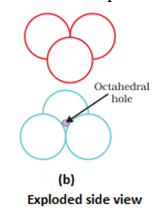
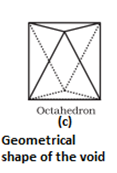
- In hexagonal close packing or cubic close packing arrangement, the octahedral and tetrahedral voids are present. The number of octahedral voids present in a lattice is equal to the number of close packed particles. The number of tetrahedral voids is twice the number of octahedral voids.
For example:
If the number of close packed particles = n
Number of particles present in octahedral voids = n
Then, the number of particles present in tetrahedral voids = 2n
- Packing efficiency: It is the percentage of total space occupied by constituent particles(atoms, molecules or ions).
- Packing efficiency for face centered unit cell =74%
- Packing efficiency for body centered cubic unit cell =68%
- Packing efficiency for simple cubic unit cell =52.4%
- Radius ratio in an octahedral void: For an atom to occupy an octahedral void, its radius must be 0.414 times the radius of the sphere.
- Radius ratio for tetrahedral void: For an atom to occupy a tetrahedral void, its radius must be 0.225 times the radius of the sphere.
- Density of a unit cell is same as the density of the substance.
- Relationship between radius of constituent particle(r) and edge length(a):
- Simple cubic unit cell: a=2r
- Face centered unit cell: a=
- Body centered unit cell: a=
- Volume of a unit cell=(edge length)³= a³
- Simple cubic unit cell: Volume=2r³
- Face centered unit cell: Volume=
- Body centered unit cell: Volume=
- Number of atoms in a unit cell(z):
- Simple cubic unit cell: z = 1
- Face centered unit cell: z = 4
- Body centered unit cell: z = 2
- Density of unit cell =
- Crystal defects are basically irregularities in the arrangement of constituent particles.
- Types of defects:
- Point defects- Point defects are the irregularities or deviations from ideal arrangement around a point or an atom in a crystalline substance.
- Line defects- Line defects are the irregularities or deviations from ideal arrange ment in entire rows of lattice points.
Types of Crystal Defects
Defects or Imperfections in crystalline solid can be divided into four groups namely line defects, point defects, volume defects and surface defects.
- Point Defect: The defect is due to a fault produced in the arrangement of a point i.e. a constituent particle like atom, ion or a molecule.
- Line Defect: The defect is due to irregularity in a complete line, a row of lattice points of the constituent.
- Surface Defect: Surface defects are the boundaries or planes that separate material into regions, with each region...
- Volume Defect: Volume defects are Voids, i.e. the absence of a number of atoms to form internal surfaces in the crystal.
What are Point Defects?
What are point defects ?
- One or more atoms of the crystal are missing from their corresponding lattice site.
- Atom(/s) is shifted from its corresponding lattice site to interstitial position in the crystal.
- Foreign atoms occupy the interstitial position in the crystal lattice.
- Original atom of the crystal is replaced by foreign atom.
- Vacancy: A vacancy is the simplest point defect and involves a missing atom within a metal.
- Interstitialcy: It is the addition of an extra atom within a crystal structure particularly if the atomic packing factor is low.
- Schottky Imperfections: These are closely related to vacancies but are found in compounds which must maintain a charge balance.
- Frenkel Defect: An ion dislodged from the lattice into an interstitial site is called Frenkel defect.
- Compositional Defects: These defects arise from impurity atom during original crystallisation. Impurity atoms considered as defects in a perfect lattice are responsible for the functioning of most semiconductor devices.
1. Stoichiometric Defect:
Fundamentally, they are of two types:
The density of a substance remains unchanged.
It happens when there is a huge difference in the size of anions and cations.
Example: ZnS and AgCl
The density of a substance remains unchanged.
It happens when there is a huge difference in the size of anions and cations.
Example: ZnS and AgCl
Schottky Defect
This kind of vacancy defects is found in Ionic Solids. But in ionic compounds, we need to balance the electrical neutrality of the compound.
An equal number of anions and cations will be missing from the compound.
It reduces the density of the substance.
In this, the size of cations and anions are of almost the same.
2. Non-Stoichiometric Defect:
Metal deficiency defect: In this, the solids have less number of metals relative to the described Stoichiometric proportion.Metal excess defect: There are two types of metal excess defect:
Metal excess defect due to the presence of extra cations at interstitial sites:
3. Impurity Defect:
- Different types of point defects:
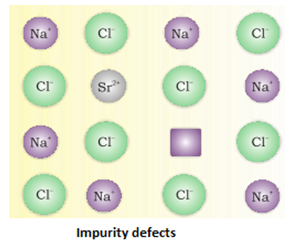
- Different types of stoichiometric defects for non- ionic solids:
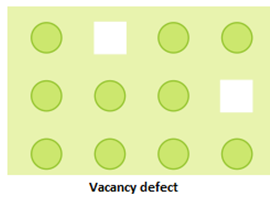
- Vacancy defect: A crystal is said to have vacancy defect when some constituent particles (atoms or molecules) are missing from its lattice site. This defect results in decrease in density of the substance.
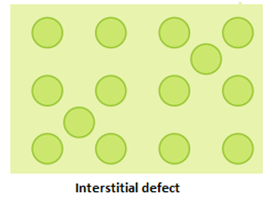
- Interstitial defect: A crystal is said to have interstitial defect when some constituent particles (atoms or molecules) occupy an interstitial site. This defect results in increase in density of the substance.
- Different types of stoichiometric defects (for ionic solids):
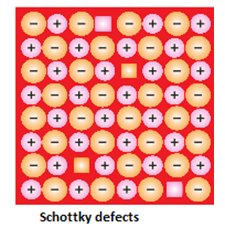
- Schottky Defects :Schottky defect in crystals is observed when equal numbers of cations and anions are missing from the lattice sites. It is important that an equal number of cations and anions are missing, otherwise the electrical neutrality of the crystal will get affected. Schottky defect is a type of vacancy defect which is found in ionic solids. It is a stoichiometry defect (thermodynamic defect), which means that such defects do not change or distort the stoichiometry of the solid. Most of the times, the Schottky defect is found in lattices having ions with nearly equal sizes. This type of defect is common in lattices like NaCl, KBr, and KCl.
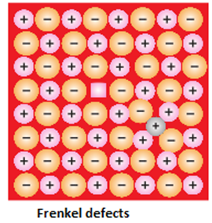
- Frenkel or dislocation defect: In this defect, the smaller ion (usually cation) is dislocated from its normal site to an interstitial site. It creates a vacancy defect at its original site and an interstitial defect at its new location. It does not change the density of the solid. Frenkel defect is shown by ionic substance in which there is a large difference in the size of ions. In ionic solids generally, the smaller ion (cation) moves out of its place and occupies an intermolecular space. In this case, a vacancy defect is created on its original position and the interstitial defect is experienced at its new position. This type of defect is common in lattices like ZnS, AgCl, AgBr and AgI.
- Different types of non-stoichiometric defects:
- Metal deficiency Defect: This defect arises because of absence of metal ions from its lattice sites. The electrical neutrality is maintained by an adjacent ion having a higher positive charge.
Metal Deficiency defects can arise due to two main reasons. They are:-
- Cation Vacancies
- Extra anions occupying interstitial states
-
Due to Cationic Vacancies
- Cationic Vacancies arise when one of the positive ions leaves the lattice site and the additional negative charge balances by receiving two positive charges instead of just one.
- Cation Vacancy defect is usually located in compounds of transition metals that have variable valency or oxidation states. FeO and FeS show this kind of metal deficiency defect.
- FeO is a crystal with a metal shortage. The metal scarcity in FeO occurs due to the fact that the constructive ion present in the crystal is smaller in size than the negative ions.
- Cationic vacancies occur when the cation is not present in its original Lattice position.
- There are defects where the lattice position of the cation is vacant but the number of cations and anions remains balanced as the missing anions and cations will both be equal or the cation will be occupying an interstitial position.
- The cationic vacancy is mainly due to the absence of the cation in the crystal which leads to an imbalance of charge.
- In order to balance this charge, the neighbouring cation gains two positive charges, one more than the usual.
- This eventually balances the charge on the crystal.
- However, there will be less number of metal ions as compared to the crystals of the vacancies, resulting in a change in the crystal’s composition.
- The defect can only occur when the metal ion has two or more oxidation states.
- Therefore, this defect is possible with the elements of d-block.
- 2.
Due to Extra Anions Occupying the Interstitial Sites
Metal Deficiency Defect also occurs due to extra anions occupying the interstitial sites.
- In this case, the additional positive charges might be in the interstitial positions.
- The additional negative charge in the lattice is balanced by the additional charges at the adjoining metal ions.
- This type of metal deficiency defect is not common as negative ions are normally very big and cannot adjust in the interstitial sites.
- Reasons for the cause of metal excess defect:
A metal excess defect is one of the defects seen in the crystal structures. The other defect is metal deficiency defect. These are the non-stoichiometric inorganic solids that contain constituent elements in non-stoichiometric ration because of the defects in their crystal structures.
- Anionic vacancies and the presence of extra cations in the interstitial sites induce metal excess defects.
- When alkali metal halides are heated in an atmosphere of vapour of the alkali metal, anion vacancies are created.
- This anion then diffuses to the crystal’s surface, where it joins newly formed metal cations.
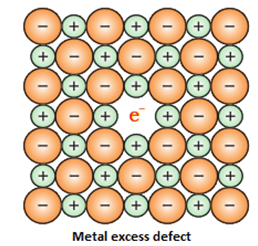
a) Anionic vacancies: A compound may have an extra metal ion if the negative ion is absent from its lattice site. This empty lattice site is called a hole. To maintain electrical neutrality this site is occupied by an electron. The hole occupied by an electron is called f-centre or Farbe Centre [*The original German Farbzentrum, where Farbe means color and zentrum means centre. ]
The anionic sites occupied by unpaired electrons are called F-centres. Such defects also impart colour to the crystals. The colour results by excitation of these electrons when they absorb energy from the visible light falling on the crystals. So the F-Centre is responsible for the colour of the compound.
Examples
F-centres give different colours as follows
- NaCl gives a yellow colour
- KCl gives a violet colour
- HCl gives pink colour
- This defect is caused due to anionic vacancies and by the presence of extra cations in the interstitial sites.
- When alkali metal halides are heated in an atmosphere of vapour of the alkali metal, anion vacancies are created. This anion is then diffuse to the surface of the crystal and combine with newly generated metal cations.
- The electron is lost by the metal atom is then diffuses the crystal and occupy the anionic vacancy site and form F-centres inside the crystal.
- These F-centres give different colours like NaCl gives a yellow colour. KCl gives a violet colour and HCl gives pink colour.
Example
When NaCl is heated in an atmosphere of Sodium (Na) vapours. The excess of Na atoms deposition in the surface of NaCl crystal Cl– ions then diffuse to the surface where the combine with Na+ ions which becomes due to losing electrons.
b) Presence of extra cations: A compound is said to have extra cations if a cation is present in the interstitial site. An electron is present in the interstitial site to maintain the electrical neutrality.
In this defect, on heating the compound, it releases extra cations. These cations occupy the interstitial sites in crystals and the same number of electrons goes to neighbouring interstitial sites. Extra cations in the interstitial site can be found in crystals that exhibit Frenkel defect such as ZnO.
Example
Zinc oxide is white at room temperature, on excess heating, it turns yellow as it loses oxygen.
The key difference between metal excess defect and metal deficiency defect is that metal excess defect is caused by anionic vacancies and extra cations in the interstitial sites whereas metal deficiency defect is caused by cationic vacancies and extra anions in the interstitial sites.
Metal excess defect and metal deficiency defect are two types of defects we can observe in crystal lattices of some substances. These defects arise due to the presence or absence of cations or anions in the crystal lattices.
Solved Example Based on Metal Deficiency Defects
Example 1: The Fe0.94O (nonstoichiometric compound) is formed when the x% of Fe2+ ions are adjusted or replaced by 2/3x% of Fe3+ ions. Determine the value of x.
Solution: x% of Fe2+ ions are taken by Fe3+ ions.
No. of Fe3+ that are replacing the Fe2+ ions is two-thirds of x, which gives us vacancies of cations.
Vacancies of cations = x - 2/3x = 1/3x.
Fe0.94O is the formula of the stoichiometric compound.
x/3 = 1-0.94= 0.06
x = 0.06 x 3
x = 0.18
x = 0.18 x100 % = 18%.
- Classification of solids based on their electrical conductivities:
a) Conductors: The solids with conductivities ranging between to are called conductors.
b) Insulators: These are the solids with very low conductivities ranging between to.
c) Semi- conductors: These are the solids with conductivities in the intermediate range from
to .
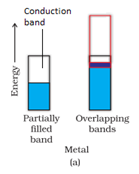
b) In case of insulators, the forbidden gap is very large and the electrons are unable to excite to the conduction band.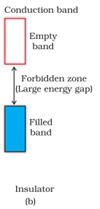
c) In case of semiconductors, forbidden gap is small. Therefore, some electrons may jump to conduction band and show some conductivity. Electrical conductivity of semiconductors increases with rise in temperature, since more electron scan jump to the conduction band.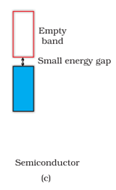
- Types of semiconductors:
a) Intrinsic semiconductors: These are those semiconductors in which the forbidden gap is small. Only some electrons may jump to conduction band and show some conductivity. They have very low electrical conductivity. Example: Silicon, germanium.
- Intrinsic Semiconductors are those which conduct electricity when the temperature is raised above room temperature. Since outside temperature can change the conductivity, the device that incorporates the mechanism of intrinsic semiconductors can pose serious problems.
- Due to this reason, scientists, after significant research and experimentation, came up with the concept of extrinsic semiconductors.
b) Extrinsic semiconductors:
Extrinsic semiconductors are those in which impurity is specifically added from the outside. The impurities are generally in the form of either trivalent or pentavalent atoms. Ambient temperature doesn’t play any role in terms of electrical conductivity. It is the impurity that determines the value of conductivity. The impurities are known as dopants while the process is known as doping. This results in Extrinsic Semiconductors also being known as Doped Semiconductors.
When an appropriate impurity is added to an intrinsic semiconductor, it is called extrinsic semi conductors. Their electrical conductivity is high.
- Doping: The process of adding an appropriate amount of suitable impurity to increase the conductivity of semiconductors is known as doping.
a) The n-type semiconductors: They are formed when silicon is doped with electron rich impurity like group 15 elements. The increase in conductivity is due to the negatively charged electrons.
Example :-When the dopants from group V of the periodic table are added to the semiconductor, it leads to the formation of an N-type semiconductor. If a pentavalent atom Arsenic (As) is added to the semiconductor which has 5 electrons in its valence shell. Four of the electrons of As will bond with the neighboring Ge atom while the remaining one electron will move freely in the semiconductor thus the ionization enthalpy which is needed to free the remaining electron of Arsenic will decrease.
It is a well known fact that there is one extra electron of Arsenic that isn’t able to form any bond in the lattice structure, due to which the semiconductor has an extra electron. This is the reason why the term donor is used here.
b) The p-type semiconductors: They are formed when silicon is doped with electron deficient impurity like group 13 elements. The increase in conductivity is due to the positively charged holes.
Example :- When the impurities in the form of trivalent atoms like Aluminium are added to the semiconductor, then it leads to the formation of a P-type Semiconductor.
On adding the trivalent Aluminium atom that has three electrons in its valence shell, all of them will form a bond with the Ge atom but one extra electron is still needed to complete the pairing of four electrons of the Ge atom. Aluminium has just three valence electrons which lead to the formation of the hole between Al and Ge.
To fill up this gap, the electron from outer orbit will be shifted to this hole but in turn, it forms a gap at its original location. It is a well-known fact that the number of holes (positive charge) is more than the number of electrons. Due to this reason, it can exhibit the property of the acceptor.
- Types of extrinsic semiconductors:
- Diode: It is a combination of n-type and p-type semiconductors and is used as a rectifier.
- Transistors: They are made by sandwiching a layer of one type of semiconductor between two layers of the other type of semi conductor. The n-p-n and p-n-p type of transistors are used to detector amplify radio or audio signals.
- The 12- 16 compounds: These compounds are formed by the combination of group 12 and group 16 compounds. They possess an average valency of 4.
- Examples – ZnS, CdS, CdSe and HgTe.
- The 13- 15 compounds: These compounds are formed by the combination of group 13 and group 15 compounds. They possess an average valency of 4.
- Examples – InSb, AlP and GaAs.
- Semiconductors generally consist of atoms like Germanium (Ge) or Silicon (Se). But once impurity is added to the semiconductor without disrupting the structure lattice, then the same semiconductor will be termed as the extrinsic semiconductor.
- The impurity added to the semiconductor is called dopant and the process of doing the same is called doping.
- The ambient temperature doesn’t play any role in the electrical conductivity. Rather, it is the impurity that determines the electrical conductivity.
- The impurities added to the semiconductor should be either from group III or V of the periodic table as the atomic size of the atoms of group III, IV, and V are approximately equal to each other. Thus, either pentavalent or trivalent impurities are added to the semiconductor.
- There will be two types of charge carriers depending on the kind of impurity added to the semiconductor which are:
- Majority charge carriers
- Minority charge carriers
- There are two types of Extrinsic semiconductors:
- N-type Semiconductor
- P-type Semiconductor
- Classification of substances based on their magnetic properties:
- Every substance has some magnetic properties associated with it. The origin of these properties lies in the electrons.
- Each electron in an atom behaves like a magnet. Its magnetic moment originates from two types of motions:
(i) its orbital motion around the nucleus and
(ii) its spin around its own axis.
- Paramagnetic substances: These are those substances which are weakly attracted by the magnetic field. It is due to presence of one or more unpaired electrons.
- Diamagnetic substances: Diamagnetic substances are weakly repelled by a magnetic field. Diamagnetism is shown by those substances in which all the electrons are paired and there are no unpaired electrons.
- Ferromagnetic substances: These are those substances which are attracted every strongly by a magnetic field.
- Anti ferromagnetic substances: They have equal number of parallel and anti parallel magnetic dipoles resulting in a zero net dipole moment.
- Ferrimagnetic substances: They have unequal number of parallel and anti parallel magnetic dipoles resulting in an at dipole moment.
Chapter 1 Solid State MCQs
Q 1.: If ‘a’ is the length of the side of a cube, the distance between the body-centered atom and one corner atom in the cube will be:
(A)
(B)
(C)
(D)
Q 2.: The number of tetrahedral and octahedral voids in a CCP array of 100 atoms are respectively:
(A) 100 and 200
(B) 200 and 100
(C) 200 and 200
(D) 100 and 100
Q 3.: Copper crystallises in a face-centered cubic lattice with edge length of 361 pm. What is the radius of copper atom ?
(A) 157 pm
(B) 181 pm
(C) 128 pm
(D) 108 pm
Q 4.: A solid ‘AB’ has NaCl structure. If the radius of cation A+ is 170 pm. Calculate the maximum possible radius of the anion B–
(A) 210.3 pm
(B) 397.4 pm
(C) 410.6 pm
(D) 347.9 pm
Q 5.: The appearance of colour in solid alkali metal halides is generally due to:
(A) F-centres
(B) Frenkel defect
(C) Schottky defect
(D) Impurity defects
Q 6.: The percentage of void space for simple cubic, body centered cubic and hexagonal close packing arrangement respectively are
(A) 48, 26, 32
(B) 26, 48, 32
(C) 48, 32, 26
(D) 32, 48, 26
Q 7.: The metal has a FCC lattice, The edge length of the unit cell is 404 pm. The density of the metal is 2.72 gm/cm3. The molar mass of the metal is:
(A) 20 gm/mol
(B) 27 gm/mol
(C) 32 gm/mol
(D) 38 gm/mol
Q 8.: The number of carbon atoms per unit cell of the diamond unit cell is:
(A) 2
(B) 4
(C) 6
(D) 8
Q 9.: Which of the following defects in the crystal lowers its density ?
(A) Schottky defect
(B) Frenkel defect
(C) F-centres
(D) Impurity defects
Q 10.: Which one of the following is a covalent crystal ?
(A) Rock Salt
(B) Ice
(C) Dry ice
(D) Quartz
Q 11.: An ionic solid, ‘AB’ crystallises in BCC lattice with edge length equal to 387 pm. The distance between two oppositely charged ions in the lattice is:
(A) 300 pm
(B) 335 pm
(C) 250 pm
(D) 200 pm
Q 12.: The metal crystallises with a FCC lattice. The edge length of unit cell is 408 pm. The diameter of the metal atom is:
(A) 288 pm
(B) 204 pm
(C) 408 pm
(D) 144 pm
Q 13.: Structure of a mixed oxide is cubic close packed (ccp). The cubic unit cell of mixed oxide is composed of oxide ions. One fourth of the tetrahedral voids are occupied by divalent metal ‘A’ and the octahedral voids are occupied by monovalent metal ‘B’. The formula of oxide is:
(A) ABO2
(B) A2BO2
(C) A2B3O4
(D) AB2O2
Q 14.: If in a crystal lattice of a compound, each corner of a cube is enjoyed by sodium, each edge of a cube has oxygen and center of cube is enjoyed by tungsten, then give its formula:
(A) Na2WO4
(B) NaWO3
(C) Na3WO3
(D) NaWO3
Q 15.: Sodium metal crystallises in BCC lattice with cell edge, 429 pm. What is the radius of sodium atom ?
(A) 286 pm
(B) 681 pm
(C) 186 pm
(D) 168 pm
Q 16.: Which of the following compounds shows both Frenkel and Schottky defects ?
(A) NaCl
(B) KCl
(C) AgCl
(D) AgBr
Q 17.: Schottky defect in crystal is observed when:
(A) Density of crystal is increased
(B) An ion leaves its normal site and occupies an interstitial site
(C) Equal number of cations and anions are missing from the lattice
(D) Unequal number of cations and anions are missing from the lattice
Q 18.: Sodium crystallises in BCC arrangement with the interfacial separation between the atom at the edge 53 pm. The density of the solid is:
(A) 1.23 gm/cc
(B) 0.48 gm/cc
(C) 4.85 gm/cc
(D) 2.42 gm/cc
Q 19.: A solid is formed by two elements P and Q. The element Q forms cubic close packing and atoms of P occupy one third of tetrahedral voids. The formula of the compound is:
(A) PQ3
(B) P2Q3
(C) P3Q
(D) P3Q2
Q 20.: Aluminium has FCC structure. The length of the unit cell is 404 pm. If the density of the metal is 2.7 g/cc, the molar mass of Al atom is:
(A) 26.80 gm/mol
(B) 27.80 gm/mol
(C) 28.46 gm/mol
(D) 26.10 gm/mol
Q 21.: The unit cell of a binary alloy composed of ‘A’ and ‘B’ metals, has a CCP structure with ‘A’ atoms occupying the corners and ‘B’ atoms occupying centres of each face of the cube. If during the crystallization of this alloy, in the unit cell two A-atoms are missed, the overall composition per unit cell is:
(A) AB3
(B) AB4
(C) AB6
(D) AB8
Q 22.: The cubic unit cell of a metal (molar mass = 63.55 gm/mol) has an edge length of 362 pm. Its density is 8.92 gm/cc. The type of unit cell is:
(A) Primitive Unit Cell
(B) Face-centered Unit Cell
(C) Body-centered Unit Cell
(D) End-centered Unit Cell
Q 23.: Which of the following statement is not correct ?
(A) The fraction of the total volume occupied by the atoms in a primitive cell is 0.48
(B) Molecular solids are generally volatile
(C) The number of carbon atoms in a unit cell diamond is 8
(D) The number of Bravais lattices in which a crystal can be categorised is 14
Q 24.: If ‘a’ stands for the edge length of the cubic systems: simple cubic, body centered cubic and face centered cubic then the ratio of radius of the spheres in these systems will be respectively,
(A) Germanium
(B) Arsenic
(C) Selenium
(D) Boron
Q 26.: Assertion (A) : No compound has both Schottky and Frenkel defects.
Reason (R) : Both defects change the density of the solid.
(A) Both A and R are correct and R is the correct explanation of A.
(B) Both A and B are correct but R is not the correct explanation of A
(C) A is correct but R is incorrect
(D) Both A and R are incorrect
Q 27.: Edge length of a cube is 400 pm, its body-diagonal would be:
(A) 566 pm
(B) 600 pm
(C) 500 pm
(D) 693 pm
Q 28.: In a FCC unit cell
(A) Octahedral voids are present at the center of the edge as well as at the center of the body.
(B) There are two tetrahedral voids along the diagonal of a cube.
(C) Both A and B
(D) There are two octahedral voids along the diagonal of a cube.
Q 29.: The fraction of total volume occupied by the atoms present in a simple cube is:
Q 30.: If AgI crystallises in zinc blende structure with iodide ions at lattice points. What fraction of tetrahedral voids is occupied by silver ions ?
(A) 25 %
(B) 50 %
(C) 75 %
(D) 100 %
Q 31.: CsBr crystallises in a BCC lattice. The unit cell length is 436.6 pm. Given that the atomic mass of Cs = 133 u and that of Br = 80 u. The density of CsBr is:
(A) 42.5 gm/cc
(B) 0.425 g/cc
(C) 8.25 gm/cc
(D) 4.25 gm/cc
Q 32.: Pure silicon doped with phosphorous, is a
(A) Metallic conductor
(B) Insulator
(C) n-type semiconductor
(D) p-type semiconductor
Q 33.: Na and Mg crystallise in BCC and FCC type crystals respectively, then the number of atoms of Na and Mg present in the unit cell of their respective crystal is:
(A) 4 and 2
(B) 9 and 14
(C) 14 and 9
(D) 2 and 4
Q 34.: A particular solid is very hard and has a high melting point. In solid state, it is a non-conductor and its melt is a conductor of electricity. Classify the solid:
(A) Metallic Solid
(B) Ionic Solid
(C) Molecular Solid
(D) Covalent or Network Solid
Q 35.: When electrons are trapped into the crystal in anion vacancy, the defect is known as:
(A) F-centres
(B) Schottky defect
(C) Frenkel defect
(D) Metal deficient defect
Q 36.: The radius of Sodium ion and Chloride ion are 95 pm and 181 pm respectively. The edge length of NaCl unit cell is:
(A) 276 pm
(B) 138 pm
(C) 552 pm
(D) 415 pm
Q 37.: Some of the polar crystals when heated produces small electrical current. This phenomenon is called _____________
(A) Pyroelectricity
(B) Ferroelectricity
(C) Piezoelectricity
(D) Anti-ferroelectricity
Q 38.: In a face centered cubic lattice, a unit cell is shared equally by how many unit cells ?
(A) 2
(B) 4
(C) 6
(D) 8
Q 39.: Which of the following unit cell having maximum number of atoms ?
(A) HCP
(B) FCC
(C) BCC
(D) Cubic
Q 40.: Which of the following statement is incorrect about crystalline solids ?
(A) Crystalline substances are anisotropic
(B) Crystalline solids have definite enthalpy of fusion.
(C) Solid SO2 is a polar molecular solid
(D) All covalent solids are poor conductor of electricity and are insulator
In the following article we shall study and discuss some of the most important problems in Solutions which are of utmost importance for the annual and Entrance examinations like OUAT, NEET, JEE, IIT etc.
Thanks for reading the article till the end.
Bye.
For Personal guidance and coaching,
- For Joining