Contents [hide]
- What are haloalkanes ( alkyl halides) ?
- Classification of haloalkanes
- Nomenclature of haloalkanes
- Isomerism in haloalkanes
- Preparation of haloalkanes
- A. Preparation of haloalkanes from alcohols :
- B. Preparation of haloalkanes from hydrocarbons :
- Markovnikov’s rule :
- Peroxide effect :
- Physical properties of haloalkanes
- Chemical properties of haloalkanes
- 1. Nucleophilic substitution reaction in haloalkanes
- 2. Elimination reaction
- Saytzeff’s rule ( or Zaitsev rule) :
- 3. Reaction of haloaklanes with metals
- 4. Reduction reaction of haloalkanes
- Chloroform (trichloromethane) (CHCl3) :
- Questions and Answers
- Questions and their Answers
- Exercise
- References
What are haloalkanes ( alkyl halides) ?
Alkyl halides or haloalkanes are the organic compounds in which halogen atom is bonded to an alkyl group. The general formula of these compounds is R – X .

Classification of haloalkanes
Haloalkanes are classified into primary, secondary and tertiary haloalkanes depending upon the number of carbon atoms to which halogen linked carbon is bonded.
1. Primary haloalkane ( 10 haloalkane) :
A haloalkane in which halogen linked carbon is bonded to none or one other carbon atom is known as primary haloalkane.
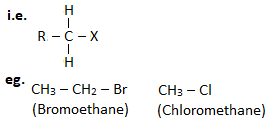
2. Secondary haloalkane ( 20 haloalkane) :
A haloalkane in which halogen linked carbon is further bonded to two other carbon atoms is known as secondary haloalkane.
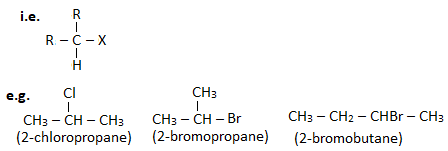
3. Tertiary haloalkane ( 30 haloalkane) :
A haloalkane in which halogen linked carbon is further bonded to three other carbon atoms is known as tertiary haloalkane.
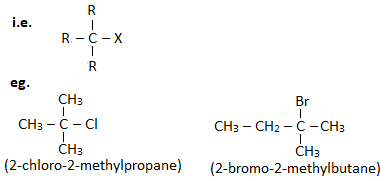
Nomenclature of haloalkanes
A. Common system :
- In common system, haloalkanes are named by adding the word halide after the name of alkyl group.
- The words n-, sec-, tert-, iso- and neo- are usually used in writing the common names.
Common name of alkyl halide is always written as two separate words.
B. IUPAC system :
- In IUPAC system, haloalkanes are named by adding prefix ‘halo-‘ before the name of parent alkane.
The IUPAC name of any monohalo alkanes is always written as one word.

Note : n = straight chain (10)
sec = 20 , tert = 30
iso = 10 ( if second last carbon contains one methyl group and no other branches)
neo = 10 ( if second last carbon contains two methyl groups and no other branches)
Q) Write the structural formula of sec-pentyl chloride, tert-pentyl chloride and neohexyl chloride. Write their IUPAC name too.
Polyhalogen compounds :
Organic compounds containing more than one halogen atom in their molecules are known as polyhalogen compounds. Eg.

Note: Vicinal dihalide : compounds that have halogens on adjacent carbon atoms.
Isomerism in haloalkanes
Haloalkanes show chain isomerism and position isomerism.
1. Chain isomerism :
Haloalkanes having 4 or more carbon atoms show isomerism in which isomers differ in the nature of carbon chain. Eg.

2. Position isomerism :
Haloalkanes having 3 or more carbon atoms show position isomerism in which isomers differ in the position of halogen atom. Eg.

Q. Write down the possible isomers of molecular formula – (i)C4H9Br (ii)C5H11Br. Give their IUPAC names and also specify them as 10, 20 and 30 haloalkanes.
Ans.

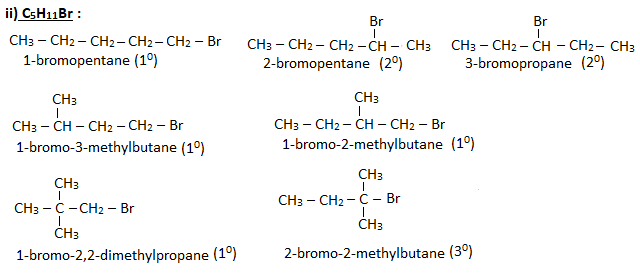
Preparation of haloalkanes
A. Preparation of haloalkanes from alcohols :
1. By the action of halogen acids :
Alcohols can be converted into haloalkanes by treating it with halogen acids in the presence of dehydrating agent such as anhydrous zinc chloride or conc. H2SO4.

Where, HX = HCl, HBr, HI
The reactivity of halogen acids follows the order :
HCl < HBr < HI
# It is because of the fact that bond dissociation energy is : HCl>HBr>HI.
Reactivity of alcohols towards this reaction is :
Primary<Secondary<Tertiary.
# It is because of the fact that greater the number of electron releasing groups on α- carbon atom of alcohol, more is the polarity of C – OH bond. Greater the polarity of C –OH bond, the more reactive is the alcohol.
Examples :

Note : The mixture of HCl and ZnCl2 is known as “Lucas reagent”.

2. By the action of phosphorus halides :
Alcohols on refluxing with phosphorus penta or trihalides give haloalkane.

For example :

Note :
H3PO3 = phosphorous acid
H3PO4 = phosphoric acid
POCl3 = phosphorus oxychloride ( IUPAC = Phosphoryl trichloride )
3. By the action of thionyl chloride :
Alcohols when refluxed with thionyl chloride in presence of pyridine gives chloroalkane.
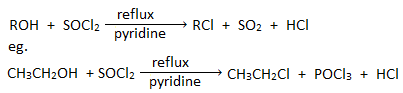
Note : SOBr2 and SOI2 are unstable, therefore bromo and iodo alkanes can not be prepared by this method.
B. Preparation of haloalkanes from hydrocarbons :
1. From alkanes :
- Alkanes when treated with limited amount of halogen in the presence of heat, light or suitable catalyst gives haloalkanes.

- If excess of halogen is used then a mixture of mono and polysubstituted products is obtained.

- Bromination also takes place in similar manner.
- Iodination is reversible thus the reaction is always carried out in the presence of oxidizing agent like HIO3, conc. HNO3, etc. The use of oxidizing agent is to oxidize ‘HI’ formed during the reaction into ‘I2’ and hence shifts the equilibrium in forward direction.

2. From alkenes : By the action of halogen acids (HCl, HBr and HI) :
Alkene reacts with halogen acids to give alkyl halide (haloalkane). Eg.

When alkene is unsymmetrical then the addition takes place according to Markovnikov’s rule.
Markovnikov’s rule :
This rule states that when an unsymmetrical reagent is added to an unsymmetrical alkene, the negative part of the reagent goes to that double bonded carbon which has lesser number of hydrogen atoms.
For example: The addition of HBr to propene gives 2- bromopropane instead of 1- bromopropane.

Peroxide effect :
When HBr is added to an unsymmetrical alkene in presence of organic peroxide, bromine goes to the double bonded carbon atom having more number of hydrogen. This phenomenon of anti- Markovnikov’s addition of HBr caused by the presence of peroxide is known as peroxide effect or anti- Markovnikov’s rule or Kharash effect.

It may be noted that peroxide effect or Kharash effect applies to the addition of HBr only and not to the addition of HCl or HI. This is because H – Cl bond is stronger than H – Br bond due to which cleavage of H – Cl bond to give Cl is unfavorable while in case of H – I the bond is cleaved very easily to give I but the I radical formed immediately combine with each other to form I2 rather than forming C – I bond.
Physical properties of haloalkanes
1.Physical state :
Lower member of alkyl halide are gaseous at room temperature (upto C5) and higher members of alkyl halide are colourless liquid or solid.
2. Boiling point :
- The boiling points of haloalkanes having same alkyl group follows the order :
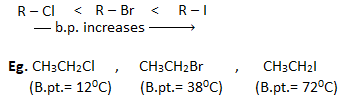
This is because with the increase in size and mass of halogen atom, the magnitude of Vander Waal’s force increases and hence boiling point also increases.
- For the alkyl halides having same halogen atoms boiling point increases with the increase in size of alkyl group. Eg.

- With the increase in branching of alkyl group, surface area decreases and magnitude of Vander Waal’s force also decreases. Hence, boiling point of isomeric alkyl halides decreases with the increase in branching of alkyl group.
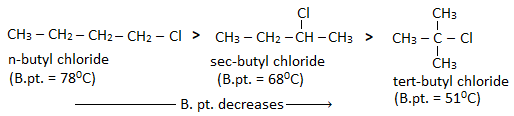
Haloalkanes have higher boiling point than alkane of comparable molecular masses. This is because haloalkanes are polar in nature and due to their polarity, a strong dipole-dipole interaction exist between the molecules of haloalkanes.

3. Solubility :
Although haloalkanes are polar in nature, they are insoluble in water because they are not able to form hydrogen bonds with water molecules. But, they are soluble in organic solvents like benzene, ether, alcohol, etc.
Chemical properties of haloalkanes
- Nucleophilic substitution reaction.
- Elimination reaction
- Reaction with metals
- Reduction reaction
1. Nucleophilic substitution reaction in haloalkanes
A nucleophilic substitution reaction is a chemical reaction which involves the displacement of leaving group by a nucleophile. In this process, the leaving group i.e. the halogen atom departs along with the bonding pair of electrons and the electrons for the formation of the new bond are furnished (provided/supplied) by the nucleophile.

It is of two types :
i. SN1 reaction :
- SN1 indicates the unimolecular nucleophilic substitution reaction.
- The rate of SN1 reaction depends only upon the concentration of the substrate.
i.e. Rate = k[Substrate]
- The reaction occurs in two steps. In first step carbocation is formed and in second step nucleophile attacks the carbocation to give substituted product.
Eg.

- Rate of the reaction is directly proportional to the stability of carbocations. Hence, the order of reactivity is : 30 > 20 > 10 haloalkanes.
ii. SN2 reaction :
- SN2 indicates the bimolecular nucleophilic substitution reaction.
- The rate of SN2 reaction depends upon the concentration of the both substrate and nucleophile.
i.e. Rate = k[Substrate][:Nu –]
- The reaction occurs in single step. . SN2 reaction occurs through a transition state as shown below: Eg.

- Rate of reaction is inversely proportional to the bulkiness of groups attached to the C atom. Hence, the order of reactivity is : 10 > 20 > 3O haloalkanes.
1. Substitution by hydroxyl group ( formation of alcohols) :
When a haloalkane is boiled with aqueous solution of KOH or moist silver oxide (Ag2O), gives alcohol.
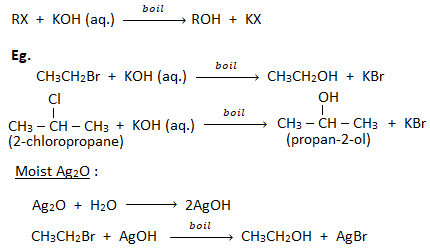
2. Substitution by cyano group ( formation of cyanides or nitriles) :
When haloalkane is treated with alcoholic KCN solution it gives alkane nitrile ( or alkyl cyanide) as the major product.

The alkyl cyanide so produced can be used as the starting material for the preparation of a number of other compounds.
- Partial hydrolysis with conc. HCl or alkaline solution of H2O2 gives amide.

- Complete hydrolysis with dil. HCl gives carboxylic acid.

- Reduction with nascent hydrogen produced by LiAlH4 (Lithium aluminium hydride), Na/alcohol or H2/Ni gives primary amine.


3. Substitution by isocyanide (i.e. – NC ) [ formation of isocyanide] :
Alkyl halide when treated with alcoholic solution of silver cyanide (AgCN) gives alkyl isocyanide.

Alkyl isocyanide on reduction gives secondary amine.


Q) Haloalkane gives alkyl cyanide with KCN while alkyl isocyanide with AgCN, why?
Cyanide ion is an ambient nucleophile which has two sites i.e. carbon and nitrogen through which it can attack alkyl halide. KCN is an ionic compound which ionizes to give cyanide ion (CN–), the negative charge of which attacks the alkyl halide to give alkyl cyanide. But AgCN is a covalent compound which does not ionize to give cyanide ion, here the lone pair of electrons present on nitrogen atom attacks alkyl halide to give alkyl isocyanide. Eg.

4. Substitution by alkoxy group [ Formation of ether] :
Alkyl halide when treated with sodium or potassium alkoxide gives ether. This reaction is known as Williamson’s ether synthesis. Eg.

5. Substitution by amino group [ Formation of amines ] :
When a mixture of haloalkane and alc. NH3 is heated it gives primary amine.

If the haloalkane is present in excess, secondary and tertiary amines are formed. Finally, tertiary amine reacts with haloalkane to give quaternary ammonium salt as the final product.

6. Substitution by nitro group (-NO2) : [Formation of nitroalkane]
Alkyl halide when treated with alcoholic solution of silver nitrite (AgNO2) gives nitroalkane.

7. Substitution by nitrite group (-O-N=O) : [Formation of alkyl nitrite]
Alkyl halide reacts with sodium or potassium nitrite to form alkyl nitrite.

Note :
Q) Haloalkane gives alkyl nitrite with KNO2 while nitroalkane with AgNO2, why?
Nitrite ion is an ambient nucleophile which has two sites i.e. oxygen and nitrogen through which it can attack alkyl halide. KNO2 is an ionic compound which ionizes to give nitrite ion [O-N=O]–, the negative charge of which attacks the alkyl halide to give alkyl nitrite. But silver nitrite is a covalent compound which does not ionize to give nitrite ion, here the lone pair of electrons present on nitrogen atom attacks alkyl halide to give nitroalkane. Eg.
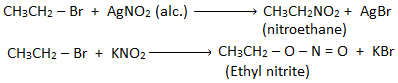
2. Elimination reaction
- When an alkyl halide is heated with alcoholic solution of KOH, then a molecule of hydrogen halide is eliminated from the haloalkane and alkene is formed. Therefore this reaction is also called dehydrohalogenation reaction. Eg.
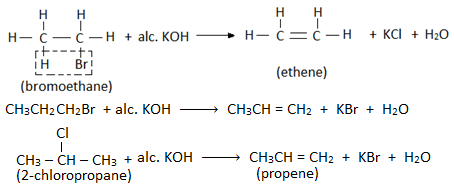
- Elimination reaction involves the removal of halogen atom of haloalkane and a hydrogen atom from the β- carbon (i.e. adjacent carbon). Therefore, this reaction is also known as β- elimination reaction.
- If two or more than two elimination products can be obtained from an alkyl halide then elimination takes place according to Saytzeff’s rule.
Saytzeff’s rule ( or Zaitsev rule) :
When two β- carbon atoms are present, then the elimination of H-atom takes place from the β- carbon atom with fewer number of H- atoms i.e. highly substituted alkene is the major product of dehydrohalogenation reaction. Eg.

3. Reaction of haloaklanes with metals
1. Reaction of haloalkanes with Na (Wurtz reaction) :
When an alkyl halide( haloalkane) is heated with sodium metal in presence of dry ether, a symmetrical alkane containing double number of carbon atoms than in haloalkane is formed. This reaction is called Wurtz reaction.

This is not good method to prepare unsymmetrical alkane because a mixture of two different haloalkanes has to be used which gives a mixture of three different alkanes.
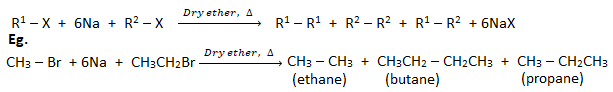
2. Reaction with magnesium :
Alkyl halide when treated with magnesium in the presence of dry ether gives alkyl magnesium halide which is known as Grignard reagent.

4. Reduction reaction of haloalkanes
Haloalkane on reduction gives the corresponding alkane. Reduction of haloalkane can be carried out by using reducing agent like (a) Zn/HCl , (b) Sn/HCl , (c) Na/ethanol , (d) H2/Ni , (e) Lithium aluminium hydride (LiAlH4), (f) Zn-Cu couple , (g) HI/Red phosphorus.
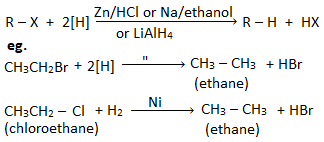
Chloroform (trichloromethane) (CHCl3) :
Preparation of Chloroform:
Chloroform is prepared by heating ethanol or acetone with aqueous bleaching powder paste. Bleaching powder paste acts as oxidizing, chlorinating and hydrolyzing agent.

From ethanol:
Step I : Oxidation :

Step II : Chlorination :

Step III : Hydrolysis :

From acetone (propanone):
Step I : Chlorination :

Step II : Hydrolysis :

Physical properties :
- Chloroform is a colourless sweet smelling liquid.
- It’s freezing point is – 630C and boiling point is 610 C.
- It is heavier than water.
- Chloroform is slightly soluble in water but soluble in ether, alcohol, etc.
- As inhaling of the vapours of chloroform induces unconsciousness therefore it can be used as anaesthetic agent for surgery.
Chemical properties :
1. Reaction with air :
In the presence of sunlight, chloroform is oxidized by air to produce highly poisonous gaseous compound called phosgene (carbonyl chloride).

Thus, chloroform is stored in a dark/coloured bottle to prevent the oxidation of chloroform into phosgene.
A small amount of ethanol is also added into the chloroform at the time of packing because ethanol converts highly poisonous phosgene ( if formed) to a non poisonous diethyl carbonate.

2. Reaction with aq. KOH solution:
When boiling with aqueous KOH solution, chloroform is hydrolysed to form potassium formate which on acidification gives formic acid.

3. Reaction with silver powder:
Chloroform when heated with silver powder gives acetylene(ethyne).

4. Reaction with primary amines (carbylamine reaction):
when chloroform is warmed with a primary amine in the presence of alcoholic KOH, an offensive(unpleasant) smell of carbylamines ( i.e. isocyanide) is obtained. This reaction is known as carbylamine reaction.

Secondary and tertiary amines do not respond to this reaction and therefore, this reaction is used as test reaction for primary amines.
5. Reaction with phenol (Riemer- Tiemann reaction):
When chloroform is heated with phenol and sodium hydroxide followed by hydrolysis, o – hydroxy benzaldehyde ( salicylaldehyde) is formed. This reaction is called Riemer – Tiemann reaction.

6. Reaction with acetone(propanone):
Chloroform reacts with acetone in presence of a base such as KOH to give chloretone.

Chloretone is used as a sleep – inducing (hypnotic) drug.
7. Reaction with HNO3 :
On heating with conc. HNO3, chloroform gives chloropicrin.

Chloropicrin is used as an insecticide and tear gas.
8. Reduction:
Chloroform on reduction with zinc dust and hydrochloric acid (i.e. acidic medium) gives methylene chloride (dichloromethane).

However, when reduced with zinc and water (i.e. neutral medium) gives acetylene (ethene).

Uses of chloroform :
- It is used as an anesthetic. It is now being replaced by other safe anesthetics because chloroform in some cases causes cardiac and respiratory failure.
- It is used as a laboratory reagent for testing primary amines.
- It is used for the preparation of chloropicrin, chloretone, salicylaldehyde, etc.
- It is used in medicines such as a cough syrups.
- It is used as preservative for biological specimens.

Questions and Answers
Questions and their Answers
1. Write the importance and limitations of Wurtz reaction.
Importance : Wurtz reaction is used to prepare the higher members of saturated hydrocarbons ( alkanes) from lower member of alkanes. Eg.

Limitations :
- Methane can not be prepared by this method.
- Tertiary alkyl halides do not undergo this reaction.
- It is not a good method to prepare unsymmetrical alkane because a mixture of two different haloalkanes has to be used which gives a mixture of three different alkanes.

2. How is Grignard reagent prepared? What precautions should be taken for the preparation of Grignard reagents?
Grignard reagent is the alkyl magnesium halide. It is organometallic compound which is represented by the general formula’ RMgX’. Eg.
CH3MgBr ( Methyl magnesium bromide)
CH3CH2MgI ( Ethyl magnesium iodide)
Preparation : Grignard reagent can be prepared by heating haloalkanes or haloarenes with magnesium in the presence of dry ether.
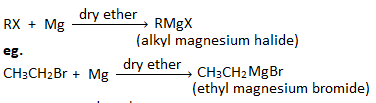
Precautions :
- Grignard reagent is very sensitive to water. When it comes in contact with water, it converts to alkane.

Therefore, during preparation of Grignard reagent there should not be the presence of water molecules i.e. all the reagents should be anhydrous and apparatus oven dried.
- There should not be naked flames nearer.
3. Identify P and Q and write their IUPAC name.

Ans.

Hence,
P = 2-bromopropane
Q = 2,3-dimethylbutane
4. Identify A and B.

Ans. A = propene
B = 2-bromopropane
5. Identify A and B and write their IUPAC name and reaction involved.

Ans.

Hence, A = Ethylmagnesium bromide
B = ethane.
6. Starting from methyl magnesium bromide, how would you prepare methane?
Methane can be prepared by boiling methyl magnesium bromide with water.
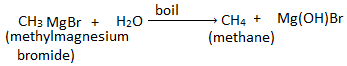
7. Identify A, B, C and D.

Ans.

Hence, A = 2-chloropropane
B = propene
C = ethanal
D = methanal
8. Identify A and B.

Ans. A = ethene and B = methanal
9. Identify A, B, C, D, E and F.

Ans.

10. How will you convert 1-bromopropane to 2-bromopropane and vice-versa.
Ans. 1- bromopropane to 2-bromopropane :
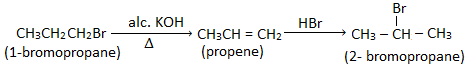
2- bromopropane to 1-bromopropane :

11) Why does chloroform does not give white precipitation with aqueous silver nitrate solution?
→ Chloroform does not give white precipitation with aqueous silver nitrate because C – Cl bond in chloroform is covalent and non – polar and does not ionize in aq. Solution to produce chloride ion (Cl –).
If chloroform is impure, precipitation will occur with aqueous AgNO3 because it may contains HCl as impurities.

12) Write the action of monohydroxy benzene with trichloromethane in presence of alcoholic caustic soda.

13) Identify the products of given reactions.

Answer :


Exercise
1. An organic compound ‘A’ on catalytic reduction gives ‘B’. ‘B’ on chlorination gives ‘C’, ‘C’ on heating with sodium metal in presence of dry ether gives ‘D’. ‘D’ on chlorination gives 2-chlorobutane as major product. Identify A, B, C and D.
2. An organic compound ‘P’ on catalytic reduction gives ‘Q’. ‘Q’ on chlorination gives ‘R’. ‘R’ on heating with sodium metal in presence of dry ether gives ‘S’. ‘S’ on chlorination gives 2-chlorobutane as major product. Give IUPAC names for P, Q, R and S.
3. A primary alkyl halide ‘A’, C4H9Br reacted with hot alcoholic NaOH to give compound ‘B’. Compound ‘B’ reacted with HBr to give ‘C’ which is an isomer of ‘A’. When ‘A’ was reacted with sodium metal it gave a compound ‘D’, C8H18 which was different than compound when n-butyl bromide was reacted with sodium. Give the structural formula and IUPAC name of ‘A’ and write all concerned reactions.
4. A chloro compound ‘A’ on reduction with Zn-Cu couple and alcohol gives the hydrocarbon ‘B’ with five carbon atoms. When ‘A’ is dissolved in ether and treated with sodium 2,2,5,5-tetramethyl hexane is obtained. Write the structure and IUPAC name of ‘A’ and ‘B’.
5. A primary haloalkane ‘P’ when allowed to react with KCN yields a compound ‘Q’,which on acidic hydrolysis gives propanoic acid. Identify ‘P’ and ‘Q’.
6. Compound ‘A’ with the molecular formula C4H9Br is treated with aq. KOH solution. The rate of this reaction depends upon the concentration of the compound ‘A’ only. When another optically active isomer ‘B’ of this compound was treated with aq.KOH solution, the rate of reaction was found to be dependent on concentration of compound and KOH both.
a. Write down the structural formula of both compounds ‘A’ and ‘B’.
b. Out of these two compounds, which one will be converted to the product with inverted configuration?
7. An organic compound (A) having molecular formula C3H7Cl on reaction with alcoholic solution of KCN gives compound B. The compound B on hydrolysis gives compound C. C on reduction with H2 / Ni gives 1-aminobutane. Identify A, B and C.
8. An organic compound ‘A’ reacts with alcoholic KOH to give ‘B’. ‘B’undergoes ozonolysis and gives two compounds ‘C’ and ‘D’ of molecular formula C3H6O. C and D are functional isomers of each other.
a. Write chemical equation for the conversion of A into C and D.
b. Write the structural formula of C and D. why are they called functional isomers?
c. What happens when hydrogen gas in presence of nickel catalyst is passed over ‘X’.
d. What is the application of ozonolysis in the organic reaction mechanism?
e. How can you prove chemically the compound X is unsaturated?
9. A secondary haloalkane X on dehydrohalogenation gives Y. Y on ozonolysis followed by hydrolysis gives ethanal and methanol as major product .
a. Identify X and Y
b. Write all chemical reactions involved
c. What happens when X is heated with sodium metal in presence of dry ether?
d. How would you distinguish X from propane?
10. An organic compound ‘A’ can be used as an anesthetic gives acetylene when heated with silver powder.
a. Write chemical reaction involved in the preparation ‘A’ from ethanol.
b. What precautions should be followed during the storage of ‘A’? Explain with proper reactions.
c. What happens when ‘A’ is heated with aq.KOH solution?
d. How would you prepare chloretone from ‘A’?
11. Differentiate between SN1 and SN2 reaction.
Convert:
- Methane to ethane
- Ethane to ethene
- Methane to ethanoic acid
- Ethanol to ethyne
- Ethane to propanamine
- Chloroethane to propan-2-ol
- 1-bromopropane to 2-bromopropane and vice-versa
Write one example of each:
- Carbylamines reaction
- Riemer-Tiemann reaction
- Dehydrohalogenation reaction
- Wurtz reaction
- SN1 reaction
- SN2 reaction
Account for the following:
- Chloroform is stored in a dark bottle filling upto the brim.
- Small amount of ethanol is added in the bottle of chloroform.
- Chloroform does not give white precipitate with aqueous silver nitrate.
- SN2 reaction gives inverted product.
- Ethanol is soluble in water but ethyl chloride is not soluble.
- Boiling point of n-butyl alcohol is higher than that of tert-butyl alcohol.
- chloroform is not used as an anesthetic these days.
- alkyl halide gives alcohol with aq. KOH while with alcoholic KOH gives alkene.
- Haloalkane reacts with KCN to give alkyl cyanide but with AgCN gives alkyl isocyanide.
References
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Morrison, R.T. , Boyd, R.N., Organic Chemistry, Sixth edition, Prentice-Hall of India Pvt. Ltd., 2008.
- March, j., Advanced Organic Chemistry, Fourth edition, Wiley Eastern Ltd. India, 2005.
- https://en.wikibooks.org/wiki/Organic_Chemistry/Haloalkanes
- https://www.sciencedirect.com/topics/chemistry/haloalkane
- https://www.organic-chemistry.org/namedreactions/wurtz-reaction.shtm

