Aromatic Compounds – Structure, Preparation, Properties and Uses of Benzene.
Contents
- What are aromatic compounds ?
- Structure of benzene :
- Kekule’s structure of benzene
- Resonance structure of benzene
- Molecular orbital structure of benzene
- Nomenclature of aromatic compounds
- The priority order of functional groups :
- Benzyl, Benzal and Benzo groups
- Huckel’s rule
- Preparation of benzene
- Chemical properties of benzene
- Electrophilic substitution reactions of benzene
- Addition reactions of benzene
- Combustion reaction of benzene
- Uses of benzene
- Benzene preparation and properties (reactions) flowchart
- Questions and their answers
- References
What are aromatic compounds ?
Benzene and those cyclic compounds that chemically behave as benzene are called aromatic compounds. Eg.

Aromatic compounds are also known as arenes (i.e. aromatic alkenes).
{ the term aromatic was derived from the Greek word ‘aroma’ meaning sweet smelling}Structure of benzene :
Kekule’s structure of benzene
Kekule, a German scientist proposed the structure of benzene for the first time. According to Kekule, all the 6 carbon atoms of benzene molecule are joint to each other by alternate single and double bond forming a hexagonal ring and a hydrogen atom is bonded to each carbon atom.

Resonance structure of benzene
The double bonds may be localized in any position and therefore following resonating structures are possible :

According to these structures, there should be three single bonds (bond length 154 pm) and three double bonds (bond length 134 pm) between carbon atoms in the benzene molecule. But actually it has been found by X- ray diffraction studies that all the carbon-carbon bonds in benzene are equivalent and have bond length 139 pm , which is intermediate between C – C (154 pm) and C = C (134 pm). Thus, the actual structure of benzene is different from both ‘A’ and ‘B’ and is a resonance hybrid of these two resonating forms.
{ Note: pm = picometre, 1pm = 10 -12 m}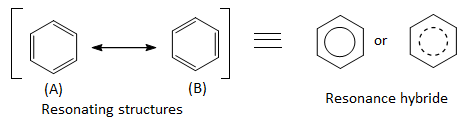
Molecular orbital structure of benzene
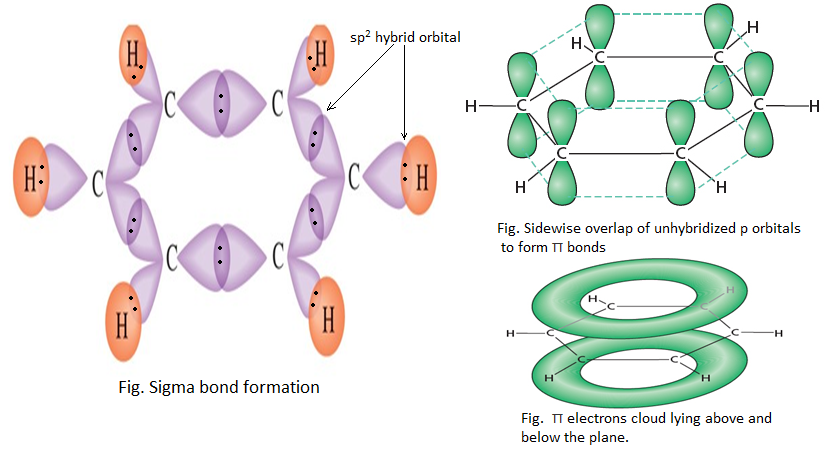
Each carbon atom in benzene has to join to three other atoms( one H and two C) and doesn’t have enough unpaired electrons to form the required number of bonds, so it needs to promote one of the 2s2 electron into empty 2pz orbital. This is called excited state configuration.

Because each carbon is only joining to three other atoms, carbon atom is sp2 hybridized using one 2s electron and two 2p electrons leaving one 2p electron unchanged.
Two of the sp2 hybrid orbitals of each of the six carbon atoms overlap with each other to form a hexagonal ring, while rest of the hybrid orbital overlaps with 1s orbital of a hydrogen atom. The unhybridized 2pz orbital of each carbon atom undergoes sidewise overlapping below and above the ring to form π bonds.
Therefore, a benzene molecule consists of twelve σ bonds and three π bonds. It shows the planar type of structure with bond angle 1200 between two carbon atoms.
Nomenclature of aromatic compounds
1. Naming of mono-substituted benzene
Monosubstituted benzenes are usually named by prefixing the name of the substituent before the word ‘ benzene’. Eg.

However, some of the monosubstituted benzenes are known by their special names.


2. Naming of disubstituted benzene
A disubstituted benzene shows isomerism ( i.e. position isomerism). The second substituent can enter into any one of the five positions of the ring. Since positions (2 and 6) and (3 and 5) are equivalent positions, there can be three isomers of the disubstituted benzenes. Eg.


- 2 and 6 positions are also indicated by prefix ortho (o-)
- 3 and 5 positions are also indicated by prefix meta (m-)
- Position ‘4’ is also indicated by prefix para (p-). Eg.


If two different substituents are present then ortho, meta and para is followed by the substituent in an alphabetical order. Eg.

If one of the substituent present in the disubstituted benzene gives the special name then disubstituted benzene is named as derivatives of that special molecule. Eg.


If both the substituent present in disubstituted benzene give the special name, then the substituent with higher priority is numbered as 1.
The priority order of functional groups :
-COOH > -SO3H > – COO- > -COX > -CONH2 > -CN > -CHO > -CO- > -OH > -NH2



3. Naming of tri or polysubstituted benzene


Note : In some cases benzene ring is considered as substituent. Eg.

Benzyl, Benzal and Benzo groups

Also see the Nomenclature of Aliphatic compounds …
Necessary conditions for any compound to show aromaticity
The necessary conditions for any compound to show aromaticity are as follows :
- The compound must be cyclic and planar and allow cyclic overlap of p-orbitals.
Note : Usually ring compounds containing upto 7 carbon atoms are planar.
- There must be complete delocalization of π – electrons.
- The compounds must contains π – electrons according to Huckel’s rule, i.e. (4n+2) π – electrons, where n = 0, 1, 2,3, 4, etc.
Huckel’s rule
Huckel’s rule states that a cyclic, planar and conjugated molecule is aromatic if it contains 4n+2 delocalized π electrons, where n = 0, 1, 2, 3, 4, etc.
Examples :
i. Benzene :
 Benzene is cyclic and planar and has cyclic overlap of p-orbitals. There are 3 double bonds i.e. 6 delocalized π – electrons, which is consistant with Huckel’s rule.
Benzene is cyclic and planar and has cyclic overlap of p-orbitals. There are 3 double bonds i.e. 6 delocalized π – electrons, which is consistant with Huckel’s rule.
i.e. 4n+2 = 6
4n= 4
n = 1(which is an integer)
Therefore, benzene is an aromatic compound. It will show aromaticity.
ii. Cyclooctatetraene :
 It is cyclic, planar and has a cyclic overlap of p-orbitals. There are 4 double bonds and 8 π – electrons which isn’t consistant with Huckel’s rule.
It is cyclic, planar and has a cyclic overlap of p-orbitals. There are 4 double bonds and 8 π – electrons which isn’t consistant with Huckel’s rule.
i.e. 4n+2 = 8
n = 3/2 (which is in fraction)
So, cyclooctatetraene isn’t an aromatic compound. It won’t show aromaticity.
iii. Cyclopentadiene :
 It is cyclic and one of the carbon atom is sp3 hybridized. Hence, the complete overlapping of p- orbitals isn’t possible. There are 4 π – electrons which isn’t consistant with Huckel’s rule.
It is cyclic and one of the carbon atom is sp3 hybridized. Hence, the complete overlapping of p- orbitals isn’t possible. There are 4 π – electrons which isn’t consistant with Huckel’s rule.
i.e. 4n+2 = 4
n = 1/2 (which is in fraction)
So, cyclopentadiene isn’t an aromatic compound. It won’t show aromaticity.
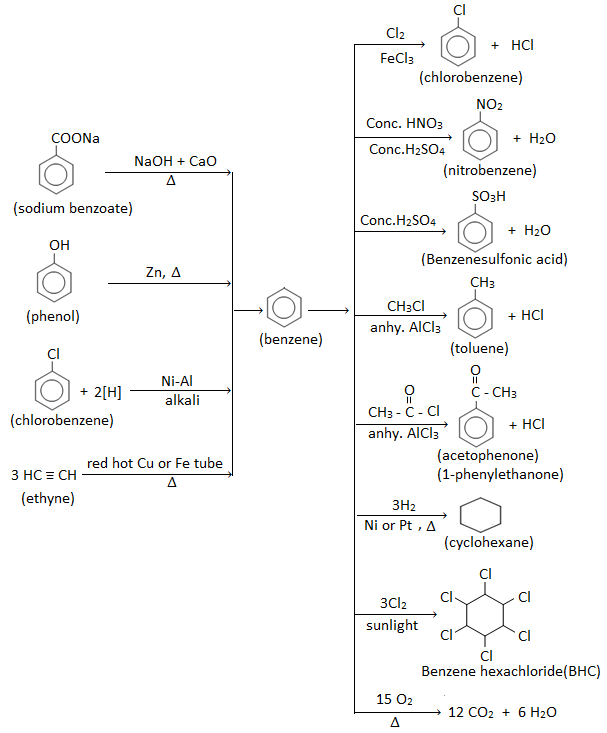
Preparation of benzene
1. From ethyne ( manufacture of benzene) :
When ethyne gas is passed through a red hot iron or copper tube, the three molecules of ethyne (acetylene) polymerize to give benzene.


2. By decarboxylation of sodium salt of benzoic acid ( Laboratory method):
Benzene can be prepared by heating sodium salt of benzoic acid ( i.e. sodium benzoate) with sodalime. This reaction is called decarboxylation reaction.

Note :

3. By reducing phenol with zinc :
When vapours of phenol are passed over heated zinc dust, benzene is produced.
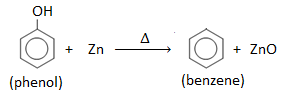
4. By the reduction of aryl halides :
Benzene can be prepared by the reduction of haloarene with nickel – aluminium alloy in the presence of alkali.

Chemical properties of benzene
Electrophilic substitution reactions of benzene
The most important/common reaction of benzene is electrophilic substitution reaction. In this reaction, an electrophile attacks the benzene and substitutes one of the hydrogen atoms of benzene ring. Eg.
1. Halogenation :
Benzene reacts with bromine in presence of ferric bromide as catalyst to give bromobenzene.

Similarly, chlorine reacts with benzene in presence of ferric chloride or AlCl3 as catalyst to give chlorobenzene.

2. Nitration : When benzene is heated with conc. HNO3 in the presence of conc. H2SO4 at about 600C gives nitrobenzene.

3. Sulphonation : When benzene is heated with conc. H2SO4 , benzene sulphonic acid is formed.

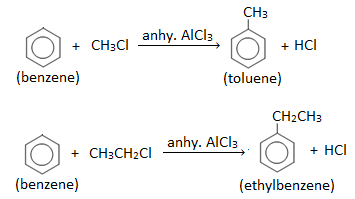
4. Friedel – Craft’s reaction :
- Friedel Craft’s alkylation : Introduction of an alkyl group ( – R ) in the benzene ring by treating benzene with an alkyl halide (R-Cl or R-Br) in the presence of anhydrous AlCl3 is known as Friedel- Craft’s alkylation. Eg.
- Friedel Craft’s acylation : Introduction of an acyl group (i.e. keto group) ( RCO- ) in the benzene ring by treating benzene with an acylating agent like acid chloride (RCOCl) or acid anhydride in the presence of anhydrous AlCl3 is known as Friedel- Craft’s acylation. Eg.


Similarly, benzene when treated with benzoyl chloride in the presence of anhydrous AlCl3 gives benzophenone.

Addition reactions of benzene
Because of unusual stability of benzene ring( due to delocalization of π – electrons), addition reactions are difficult to take place. However due to presence of three double bonds, under proper condition (drastic condition)(i.e high temperature and pressure) addition reaction takes place.
- Addition of hydrogen : When benzene vapour is heated with hydrogen gas in presence of nickel or platinum catalyst, cyclohexane is formed.
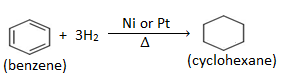
- Addition of halogen : Benzene adds three molecules of chlorine in presence of sunlight ( UV light) to give benzene hexachloride (BHC).

BHC is extensively used in agriculture as pesticide under the trade name gammexane or lindane or 666.
Combustion reaction of benzene
Benzene burns in air with sooty flame to give carbondioxide and water.
![]()
Uses of benzene
- It is used as a starting material for the preparation of varieties of aromatic compounds which are used for the manufacture of dyes, drugs, perfumes, explosives, etc. ( Eg. benzene is used for making toluene which is needed for making TNT.)
- It is used as a solvent for the extraction of fat and oil.
- It is used as a fuel for automobiles in the name of benzol. {It is used in gasoline to increase the octane rating of gasoline.}
- It is used for dry cleaning of woolen clothes.
- It is used for making phenol needed for producing Bakelite.
Benzene preparation and properties (reactions) flowchart
Questions and their answers
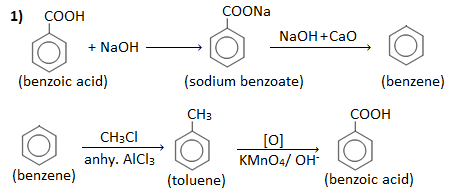
Q ) How will you convert:
1. Benzoic acid into benzene and vice-versa.
2. Phenol to toluene.
3. Phenol to acetophenone.
4. Toluene into benzene.
Answer :
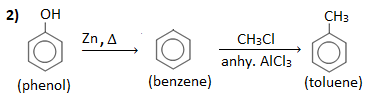


Q) Identify X and Y :

Answer :

Therefore, compound ‘X’ is benzene and compound ‘Y’ is toluene.
Q) Is cycloheptatriene an aromatic compound? Give reason.
 cycloheptatriene seems to follow Huckel’s rule i.e. 4n + 2 = 6 , or n = 1 but the π – electrons system is not delocalized due to presence of a sp3 hybridized carbon in the ring and hence does not behave as an aromatic compound.
cycloheptatriene seems to follow Huckel’s rule i.e. 4n + 2 = 6 , or n = 1 but the π – electrons system is not delocalized due to presence of a sp3 hybridized carbon in the ring and hence does not behave as an aromatic compound.
Q) Identify A to L.

References
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Morrison, R.T. , Boyd, R.N., Organic Chemistry, Sixth edition, Prentice-Hall of India Pvt. Ltd., 2008.
- https://pubchem.ncbi.nlm.nih.gov/compound/Benzene
- https://www.britannica.com/science/benzene
- https://www.chemguide.co.uk/basicorg/conventions/names3.html
