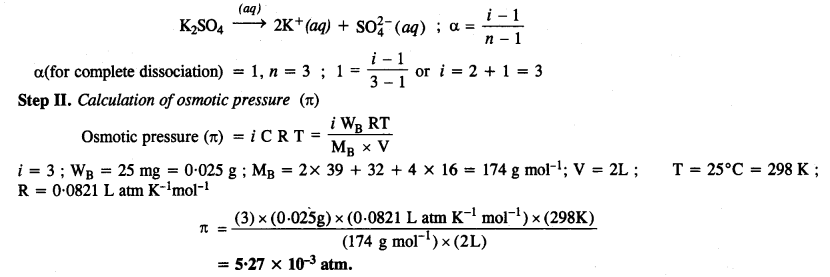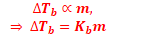Chapter 2 : Solutions
- Solutions: Solutions are the homogeneous mixtures of two or more than two components.
- Binary solution: A solution having two components is called a binary solution.
- Components of a binary solution.
It includes solute and solvent.- When the solvent is in solid state, solution is called solid solution.
- When the solvent is in liquid state, solution is called liquid solution.
- When the solvent is in gaseous state, solution is called gaseous solution.
- Concentration: It is the amount of solute in given amount of solution.
- Mass by volume percentage (w/v): Mass of the solute dissolved in 100 mL of solution.
- Molality (m) is the number of moles of solute present in 1kg of solvent.
- Molarity (M) is the number of moles of solute present in 1L of solution.
- Normality (N) is the number of gram equivalent of solute dissolved per litre of solution.
- Solubility: It is the maximum amount that can be dissolved in a specified amount of solvent at a specified temperature.
- Saturated solution: It is a solution in which no more solute can be dissolved at the same temperature and pressure.
- In a nearly saturated solution if dissolution process is an endothermic process, solubility increases with increase in temperature.
- In a nearly saturated solution if dissolution process is an exothermic process, solubility decreases with increase in temperature.
- Henry’s Law: It states “at a constant temperature the solubility of gas in a liquid is directly proportional to the pressure of gas”. In other words, “the partial pressure of gas in vapour phase is proportional to the mole fraction of the gas in the solution”.
- When a non-volatile solute is dissolved in a volatile solvent, the vapour pressure of solution is less than that of pure solvent.
- Raoult’s law: It states that “for a solution of volatile liquids the partial vapour pressure of each component in the solution is directly proportional to its mole fraction”.
- Using Dalton’s law of partial pressure the total pressure of solution is calculated.

- Comparison of Raoult’s law and Henry’s law: It is observed that the partial pressure of volatile component or gas is directly proportional to its mole fraction in solution. In case of Henry’s Law the proportionality constant is KH and it is different from p10 which is partial pressure of pure component. Raoult’s Law becomes a special case of Henry’s Law when KH becomes equal to p10 in Henry’s law.
- Classification of liquid–liquid solutions: It can be classified into ideal and non-ideal solutions on basis of Raoult’s Law.
- Ideal solutions:

- The solutions that obey Raoult’s Law over the entire range of concentrations are known as ideal solutions.
- The intermolecular attractive forces between solute molecules and solvent molecules are nearly equal to those present between solute and solvent molecules i.e. A-A and B-B interactions are nearly equal to those between A-B.

- Non-ideal solutions:
- When a solution does not obey Raoult’s Law over the entire range of concentration, then it is called non-ideal solution.
- The intermolecular attractive forces between solute molecules and solvent molecules are not equal to those present between solute and solvent molecules i.e. A-A and B-B interactions are not equal to those between A-B
- Types of non- ideal solutions:
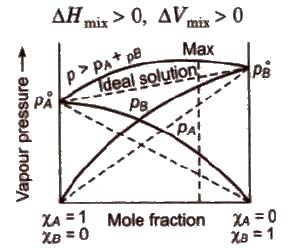
- Non ideal solution showing positive deviation
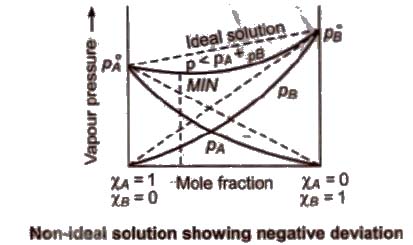
- Non ideal solution showing negative deviation
- Non ideal solution showing positive deviation
- The vapour pressure of a solution is higher than that predicted by Raoult’s Law.
- The intermolecular attractive forces between solute-solvent molecules are weaker than those between solute-solute and solvent-solvent molecules i.e., A-B < A-A and B-B interactions.
- Non ideal solution showing negative deviation
- The vapour pressure of a solution is lower than that predicted by Raoult’s Law.
- The intermolecular attractive forces between solute-solvent molecules are stronger than those between solute-solute and solvent-solvent molecules i.e. A-B > A-A and B-B interactions.
- Azeotropes:
- These are binary mixtures having same composition in liquid and vapour phase and boil at constant temperature. Liquids forming azeotrope cannot be separated by fractional distillation.
- Types of azeotropes: There are two types of azeotropes namely,
- Minimum boiling azeotrope
- Maximum boiling azeotrope
- The solutions which show a large positive deviation from Raoult’s law form minimum boiling azeotrope at a specific composition.
- The solutions that show large negative deviation from Raoult’s law form maximum boiling azeotrope at a specific composition.
- Colligative properties:
- The properties of solution which depends on only the number of solute particles but not on the nature of solute are called colligative properties.
- Types of colligative properties: There are four colligative properties namely,
- Relative lowering of vapour pressure
- Elevation of boiling point
- Depression of freezing point
- Osmotic pressure
Relative lowering of vapour pressure
- Lowering of vapour pressure: The difference in the vapour pressure of pure solvent
and solution
represents lowering in vapour pressure
.
- Relative lowering of vapour pressure: Dividing lowering in vapour pressure by vapour pressure of pure solvent is called relative lowering of vapour pressure
- Relative lowering of vapour pressure is directly proportional to mole fraction of solute. Hence it is a colligative property
Elevation of boiling point:
The difference in boiling points of solution and pure solvent
is called elevation in boiling point
- For a dilute solution elevation of boiling point is directly proportional to molal concentration of the solute in solution. Hence it is a colligative property.
Depression of freezing point:
- The lowering of vapour pressure of solution causes a lowering of freezing point compared to that of pure solvent. The difference in freezing point of the pure solvent
and solution
is called the depression in freezing point.
- For a dilute solution depression in freezing point is a colligative property because it is directly proportional to molal concentration of solute.
- Osmosis: The phenomenon of flow of solvent molecules through a semi permeable membrane from pure solvent to solution is called osmosis.
- Osmotic pressure:
- The excess pressure that must be applied to solution to prevent the passage of solvent into solution through a semipermeable membrane is called osmotic pressure.
- Osmotic pressure is a colligative property as it depends on the number of solute particles and not on their identity.
- For a dilute solution, osmotic pressure () is directly proportional to the molarity (C) of the solution i.e. = CRT
- Osmotic pressure can also be used to determine the molar mass of solute using the equation
- Isotonic solution: Two solutions having same osmotic pressure at a given temperature are called isotonic solution.
- Hypertonic solution: If a solution has more osmotic pressure than other solution it is called hypertonic solution.
- Hypotonic solution: If a solution has less osmotic pressure than other solution it is called hypotonic solution.
- Reverse osmosis: The process of movement of solvent through a semipermeable membrane from the solution to the pure solvent by applying excess pressure on the solution side is called reverse osmosis.
- Colligative properties help in calculation of molar mass of solutes.
- Abnormal molar mass: Molar mass that is either lower or higher than expected or normal molar mass is called as abnormal molar mass.
- Van’t Hoff factor: Van’t Hoff factor (i)accounts for the extent of dissociation or association.
- Value of i is less than unity in case solute undergo association and the value of i is greater than unity in case solute undergo dissociation.
- Inclusion of van’t Hoff factor modifies the equations for colligative properties as:
Solution is a homogeneous mixture of two or more substances in same or different physical phases. The substances forming the solution are called components of the solution. On the basis of number of components a solution of two components is called binary solution.
Solute and Solvent
In a binary solution, solvent is the component which is present in large quantity while the other component is known as solute.
Classification of Solutions
(A) Following types of solutions are seen on the basis of physical state of solute and solvent.
[if water is used as a solvent, the solution is called aqueous solution and if not, the solution is called non-aqueous solution.]
(B) Depending upon the amount of solute dissolved in a solvent we have the following types of solutions:
(ii) Saturated solution A solution in which no solute can be dissolved further at a given temperature is called a saturated solution.
(iii) Supersaturated solution A solution which contains more solute than that would be necessary to saturate it at a given temperature is called a supersaturated solution.
Solubility
The maximum amount of a solute that can be dissolved in a given amount of solvent (generally 100 g) at a given temperature is termed as its solubility at that temperature.
The solubility of a solute in a liquid depends upon the following factors:
(i) Nature of the solute
(ii) Nature of the solvent
(iii) Temperature of the solution
(iv) Pressure (in case of gases)
Henry’s Law
- Greater the value of KH, higher the solubility of the gas.
- The value of KH decreases with increase in the temperature.
- Thus, aquatic species are more comfortable in cold water [more dissolved O2] rather than Warm water.
Applications
1. In manufacture of soft drinks and soda water, CO2 is passed at high pressure to increase its solubility.
2. To minimize the painful effects (bends) accompanying the decompression of deep sea divers. O2 diluted with less soluble. He gas is used as breathing gas.
3. At high altitudes, the partial pressure of O2 is less then that at the ground level. This leads to low concentrations of O2 in the blood of climbers which causes ‘anoxia’.
Concentration of Solutions
The concentration of a solution is defined as the relative amount of solute present in a solution. On the basis of concentration of solution there are two types of solutions.
(i) Dilute solution
(ii) Concentrated solution
Methods of Expressing Concentration of Solutions
Various expression for the concentrations of solutions can be summarized as
(i) Percentage by weight (w / w %) It is defined as the amount of solute present in 100 g of solution.
w / w % = weight of solute / weight of solution * 100%

w / V % = weight of solute / Volume of solution * 100
or the volume of solute present in 100 mL of solution.
u / V % = volume of solute / volume of solution * 100
(iii) Mole fraction (x) It is defined as the ratio of the number of moles of a component to the total number of moles of all the components. For a binary solution, if the number of moles of A and B are nA and nB respectively, the mole fraction of A will be
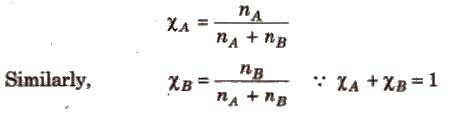
(iv) Parts per million (ppm) It is defined as the parts of a component per million parts (106) of the solution. It is widely used when a solute is present in trace quantities.
ppm = number of parts of the component / total number of parts of all the components * 106
(v) Molarity (M) It is the number of moles of solute present in 1L(dm3) of the solution.
M = number of moles of solute / volume of solution (L)
M = mass of solute (in gram) * 1000 / mol. wt. of solute x volume of solution (in mL)
Molarity varies with temperature due to change in volume of solution.
[When molarity of a solution is 1 M, it is called a molar solution. 0.1 M solution is called a decimolar solution while 0.5 M solution is known as semi molar solution]
Molarity = Percent by mass * density * 10 / molecular weight
Dilution law, M1 V1 = M2 V2 (for dilution from volume V1 to V2)
For reaction between two reactants, M1 V1 / n1 = M2 V2 / n2
where, n1 and n2 arc stoichiometric coefficient in balanced equation.
(vi) Molality (m) It is the number of moles of solute per kilogram of the solvent.
Molality = mass of solute in gram * 1000 / mol. wt. of solute * mass of solvent (in g)
Molality is independent of temperature.
[When solvent used is water, a molar (1 M) solution is more concentrated than a molal (1 M) solution.]
(vii) Normality (N) The number of gram equivalents of solute present in 1 L of solution.
Normality = number of grams – equivalent of solute / volume of solution in L
Number of gram-equivalents of solute = mass of solute in gram / equivalent weight
[Relationship between normality and molarity N x Eq. weight = M x mol. weight ]
If two solutions of the same solute having volumes and molarities V1, M1 and V2, M2 are mixed, the molarity of the resulting solution is
 To dilute V1 mL of a solution having molarity M1 to molarity M2 up to the final volume V2 mL, the volume of water added is
To dilute V1 mL of a solution having molarity M1 to molarity M2 up to the final volume V2 mL, the volume of water added is

(viii) Formality (F) It is the number of formula weights of solute present per litre of the solution.
Formality = moles of substance added to solution / volume of solution (in L))
(ix) Mass fraction Mass fraction of any component in the solution is the mass of that component divided by the total mass of the solution.
Molality, mole fraction and mass fraction are preferred over molarity, normality, etc., because former involve weights which do not change with temperature.
(x) Demal (D) It represents one mole of solute present in 1L of solution at O°C.
It is defined as "A unit of concentration, equal to the concentration of a solution in which 1 gram-equivalent of solute is dissolved in 1 cubic decimeter of solvent".
Raoult’s Law
The Raoult’s law states “For a solution of two volatile liquids, the vapour pressure of each liquid in the solution is less than the respective vapour pressure of the pure liquids and the equilibrium partial vapour pressure of the liquid is directly proportional to its mole fraction.
For a solution containing two liquids A and B, the partial vapour pressure of liquid A is
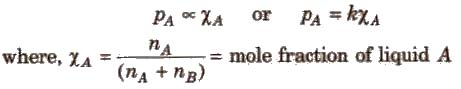 The proportionality constant is obtained by considering the pure liquid when χA = 1 then k = P°A, the vapour pressure of pure liquid, hence
The proportionality constant is obtained by considering the pure liquid when χA = 1 then k = P°A, the vapour pressure of pure liquid, hence
Konowaloff Rule
This is an empirical rule which states that in the vapor over a liquid mixture there is a higher proportion of that component which, when added to the liquid, raises its vapor pressure, than of other components.
At any fixed temperature, the vapour phase is always richer in the more volatile component as compared to the solution phase. In other words, mole fraction of the more volatile component is always greater in the vapour phase than in the solution phase.
The composition of vapour phase in equilibrium with the solution is determined by the partial pressure of components. If Y1 and Y2 are the
components 1 and 2 respectively in the vapour phase then, using Dalton’s law of partial pressure,
p1 = Y1 x Ptotal
p2 = Y2 x Ptotal
Ideal Solutions
Those solutions in which solute-solute (B-B) and solvent-solvent (A-A) interactions are almost similar to solvent solute (A-B) interactions are called ideal solutions. These solutions satisfy the following conditions :
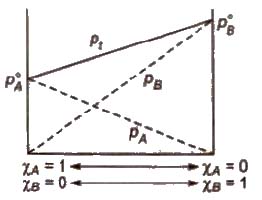
(i) Solution must obey Raoult’s law, i.e.,
(iii) ΔVmix = 0 (No expansion or contraction on mixing)
Some solutions behave like nearly ideal solutions, e.g., benzene + toluene. n-hexane + n-heptane, ethyl iodide + ethyl bromide, chlorobenzene + bromobenzene.
Non-ideal Solutions
Those solutions which shows deviation from Raoult’s law is called non-ideal solution.
For such solutions,
ΔHmix ≠ 0
ΔVmix ≠ 0
(a) Non-ideal solutions showing positive deviation In such a case, the A – B interactions are weaker than A – A or B – B interactions and the observed vapour pressure of each component and the total vapour pressure are greater than that predicted by Raoult’s law.
For such solutions
(b) Non-ideal solution showing negative deviation In such a case, the A – B interactions are stronger than A – A or B – B interactions and the observed vapour pressure of each component and the total vapour pressure are lesser than that predicted by Raoult’s law.
Azeotropic Mixture
A mixture of two liquids which boils at a particular temperature like a pure liquid and distils over in the same composition is known as constant boiling mixtures. These are formed by non-ideal solutions.
(i) Minimum boiling azeotropes are formed by those liquid pairs which show positive deviation from ideal behaviour. Such azeotropes have boiling points lower than either of the components, e.g., C2H5OH (95.57%) + H2O (4.43%)(by mass).
(ii) Maximum boiling azeotropes are formed by those liquid pain; which show negative deviation from ideal behaviour. Such azeotropes have boiling points higher than either of the components. e.g., H2O(20.22O%)+ HCl (79.78%] by mass.
Colligative Properties : Derivations
Colligative properties are those properties which depends only upon the number of solute particles in a solution irrespective of their nature.
Relative Lowering of Vapour Pressure
It is the ratio of lowering in vapour pressure to vapour pressure of pure solvent. The relative lowering in vapour pressure of solution containing a nonvolatile solute is equal to the mole fraction of solute in the solution.
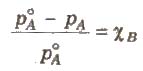
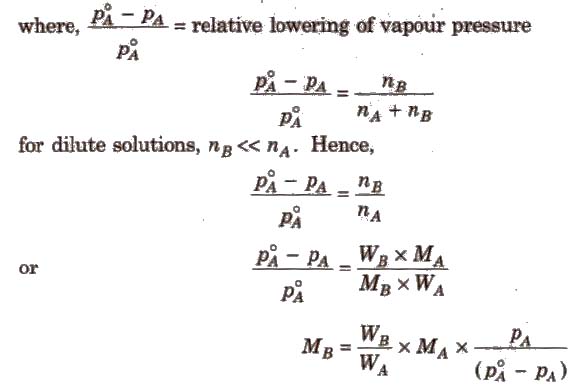
Above expression is used to find the molecular weight of an unknown solute dissolved in a given solvent. Where, WB and WA = mass of Solute and solvent respectively. MB and MA = molecular weight of solute and solvent respectively.
Ostwald and Walker method is used to determine the relative lowering of vapour pressure.
Elevation in Boiling Point (ΔTb)
Boiling point of a liquid is the temperature at which its vapour pressure becomes equal to the atmospheric pressure. As the vapour pressure of a solution containing a nonvolatile solute is lower than that of the pure solvent, it boiling point will be higher than that of the pure solvent as shown in figure. The increase in boiling point is known as elevation in boiling point, ΔTb

ΔTb = Tb – T°b
ΔTb = Kb m (where; m = molality)
Kb is molal elevation constant or ebullioscopic constant. Molecular mass of solute can be calculated as
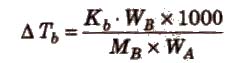
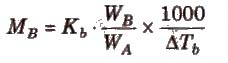
where, WB and WA = mass of solute and solvent respectively.
Kb has units of K / m or K kg mol-1, for water, Kb = 0.52 K kg mol-1
The boiling point elevation of a solution is determined by
(i) Lands Berger's method
(ii) Cottrell’s method
Depression in Freezing Point (ΔTf)
Freezing point of a liquid is the temperature at which vapour pressure of the solvent in its liquid and solid phase become equal. As we know that vapour pressure of solution containing non-volatile solute is lower than that of pure solvent, solid form gets separated out at a lower temperature as shown in the figure.
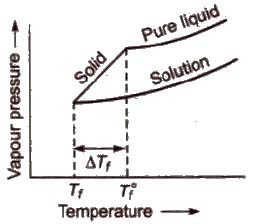
This decrease in freezing point of a liquid is known as depression in freezing point.
Depression in freezing point (ΔTf) = T°f – Tf
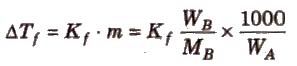
To find molecular mass of solute,

where, Kf is molal depression constant or cryoscopic constant.
Kf has units of K / m or K kg mol-1.
Ethylene glycol is usually added to water in the radiator to lower its freezing point. It is called antifreeze solution.
[Common salt (NaCl) and anhydrous CaCl₂ are used to clear snow on the roads because they depress the freezing point of water. The freezing point depression is determined by Beckmann method or Rast method.]
Calculations of molal elevation constant (Kb) and molal depression constant (Kf)
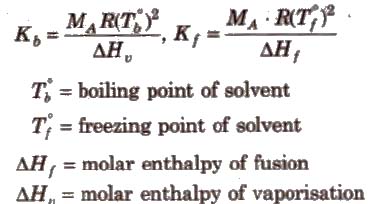
Osmotic Pressure (π)
Osmosis is the phenomenon of spontaneous flow of the solvent molecules through a semipermeable membrane from pure solvent to solution or from a dilute solution to concentrated solution. It was first observed by Abbe Nollet.
Some natural semipermeable membranes are animal bladder, cell membrane etc.
CU2[Fe(CN)6]is an artificial semipermeable membrane which does not work in non-aqueous solutions as it dissolves in them.
Osmosis may be
(i) Exosmosis It is outward flow of water or solvent from a cell through semipermeable membrane.
(ii) Endosmosis It is inward flow of water or solvent from a cell through a semipermeable membrane.
The hydrostatic pressure developed on the solution which just prevents the osmosis of pure solvent into the solution through a semipermeable membrane is called osmotic pressure.
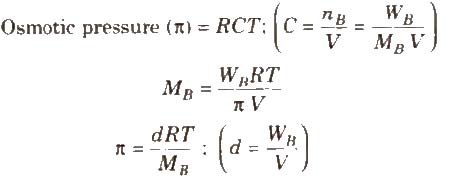
where, d = density, R = solution constant,
T = temperature, MB = molar mass of solute
Osmotic pressure can be determined by anyone of the method listed below
(i) Pfeffer’s method
(ii) Berkeley and Hartley’s method (very good method)
(iii) Morse and Frazer’s method
On the basis of osmotic pressure, -the solution can be
(i) Hypertonic solution A solution is called hypertonic if its osmotic pressure is higher than that of the solution from which it is separated by a semipermeable membrane.
When a plant cell is placed in a hypertonic solution, the fluid from the plant cell comes out and cell shrinks, this phenomenon is called plasmolysis.
(ii) Hypotonic solution A solution is called hypotonic if its osmotic pressure is lower than that of the solution from which it is separated by a semipermeable membrane.
(iii) Isotonic solution Two solutions are called isotonic if they exert the same osmotic pressure. These solutions have same molar concentration. 0.91% solution of pure NaCl is isotonic with human RBC’s.
Two solutions are isotonic if they have the same molar concentration, e.g., if x % solution of X is isotonic with y % solution of Y, this means molar concentration of X = Molar concentration of Y
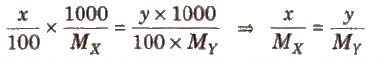
Osmotic pressure method is the best method for determining the molecular masses of polymers since observed value of any other colligative property is too small to be measured with reasonable accuracy.
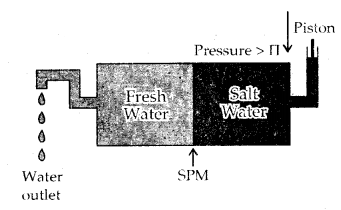
Reverse osmosis When the external pressure applied on the solution is more than osmotic pressure, the solvent flows from the solution to the pure solvent, I which is called reverse osmosis. Desalination of sea water is done by reverse Osmosis.
Abnormal Molecular Masses
In some cases, observed colligative properties deviate from their normal calculated values due to association or dissociation of molecules. As we know,
Colligative property ∝ 1 / MB
lienee, higher and lower values of molar mass is observed in case of association and dissociation respectively, e.g., in benzene, acetic acid gets associated, so, its observed molecular mass is 120. Similarly KCI undergoes dissociation in aqueous solution, so its observed molecular mass is 37.25.
These observed values are corrected by multiplying with van’t Hoff factor (i).
van’t Hoff Factor (i)
It is the ratio of observed value of colligative property to the calculated value of colligative property.
i = observed value of colligative property / calculated value of colligative property
or i = normal molecular mass / observed molecular mass
or i = number of particles after association or dissociation / number of particles initially
So to correct the observed value of molar mass, van’t Hoff factor (i) must be included in different expressions for colligative properties.
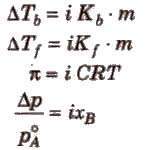
Degree of Dissociation (α) and van’t Hoff Factor (i)
(i) If one molecule of a substance gets dissociated into n particles or molecules and α is the degree of dissociation then
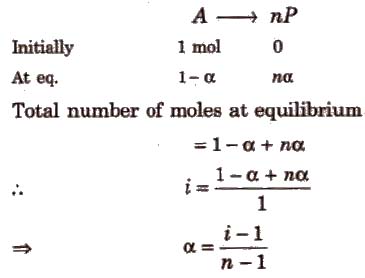
Degree of Association (α) and van’t Hoff Factor (i)
If n molecules of a substance A associate to form An and α is the degree of association then
van’t Hoff factor (i) > 1 for solutes undergoing dissociation and it is < 1 for solutes undergoing association.

Ideal and Non-Ideal Solution: A solution that obeys Raoult’s Law at all concentrations and at all temperatures is called an Ideal Solution. Herein the magnitude of solute-solvent interactions is the same as the magnitude and solvent of the solute-solute interaction- solvent interactions magnitude in the two components.
For ideal solutions enthalpy on mixing remains the same,
i.e., ΔHMix = 0
For such solutions, there is no change in volume on mixing the two components
i.e., ΔVMix = 0
Non-Ideal Solutions are those which do not obey Raoult’s Law: For such solutions, the magnitude of solute-solvent interactions is either greater than or less than the magnitude of solute-solute or solvent-solvent interactions in the pure components.
Additionally, during solution formation either heat is evolved or absorbed.
i.e.; ΔHMix = +Ve
or
ΔHMix = -Ve [or ΔHMix ≠ 0]
Moreover, there is a volume change in mixing
ΔHMix ≠ 0
Examples of ideal solutions:
- n-hexane + n-heptane
- Benzene + Toluene
- Chlorobenzene + bromobenzene
- CCl4 + SiCl4
- CH3OH + C2H5OH.
Examples of Non-ideal solutions:
| With Positive deviations | With Negative deviations |
| 1. acetone and ethanol | 1. Water + HCl |
| 2. Acetone + Benzene | 2. Water + HNO3 |
| 3. Acetone + CCl4 | 3. CHCl3 and acetone |
| 4. Benzene + CCl4 | 4. Acetic acid + pyridine |
| 5. Water + ethyl alcohol | 5. Acetone + aniline |
| 6. Cyclohexane + ethanol | 6. Benzene + CHCl3 |
Reverse Osmosis and water purification: The direction of Osmosis can be reversed if a pressure larger than the osmotic pressure is applied to the solution, side. That is, now the pure solvent flows out of the solution through the semi-permeable membrane (SPM).
Solutions : Revision at a glance
1. A solution is a homogeneous mixture of two or more chemically non-reacting substances.
The components of a solution generally cannot be separated by filtration, settling or centrifuging.
2. A solution may be classified as solid, liquid or a gaseous solution.
3. Solubility is defined as the amount of solute in a saturated solution per 100g of a solvent.
4. The solubility of a gas in a liquid depends upon
(a) the nature of the gas(solute) and the nature of the liquid(solvent),
(b) the temperature of the system (In Equilibrium), and
(c) the pressure of the gas.
5. The effect of pressure on the solubility of a gas in a liquid is governed by Henry’s Law.
It states that the solubility of a gas in a liquid at a given temperature in directly proportional to the partial pressure of the gas.
Mathematically, p = KнX
where p is the partial pressure of the gas; and X is the mole fraction of the gas in the solution and KH is Henry’s Law constant.
6. The vapour pressure of a liquid is the pressure exerted by its vapour when it is in dynamic equilibrium with its liquid, in a closed container.
or P = P° X🇦
8. A solution which obeys Raoult’s Law at all concentrations and temperatures is known as an ideal solution.
9. Characteristics of an ideal solution:
(a) ∆sol V = 0, i.e., there is no change in volume when an ideal solution is formed.
(b) ∆sol H= 0; i.e., heat is neither evolved nor absorbed during the formation of an ideal solution.
10. (a) The solution shows positive deviation from Raoult’s Law if its vapour pressure is higher than that predicted by Raoult’s Law.
(b) The solution shows negative deviation if its vapour pressure is lower than that predicted by Raoult’s Law.
11. Colligative properties of solutions are those properties which depend only upon the number of solute particles in the solution and not on their nature. Such properties are
(a) Relative lowering in vapour pressure,
(b) Elevation of boiling point,
(c) Depression of freezing point and
(d) Osmotic pressure.
12. Relative lowering of Vapour Pressure
Relative lowering of Vapour Pressure =
Thus, according to Raoult’s Law, the relative lowering of vapour pressure of a solution is equal to the mole fraction of the solute.
13. For a dilute solution, the elevation in boiling point is found to be proportional to the molality of the solution,
where ∆Tb is the elevation in boiling point, ‘m’ is the molality and Kb is the Molal elevation constant or ebullioscopic constant..
14. The depression in freezing point (∆Tf) is proportional to the molality of the solution.

where Kf is molal depression constant or cryoscopic constant (freezing point depression constant).
15. The spontaneous flow of solvent molecules from a solution of lower concentration into a solution of higher concentration when they are separated by a perfect semipermeable membrane is called osmosis.
where n is the osmotic pressure of the solution,
C is the concentration of solution
nB is the number of moles of solute,
V is the volume of the solution in litres,
R is the gas constant, and T is the temperature on the Kelvin scale.
17. Isotonic solutions are those solutions which have the same osmotic pressure. Also they have same molar concentration.
For isotonic solutions, π1 = π2 Also, C1 = C2
19. In case of association, observed molar mass being more than the normal, the factor i has a value less than one. But in case of dissociation, the van’t Hoff factor is greater than one because the observed molar mass has a lesser value.
20. In case of solutes which do not undergo any association or dissociation in a solvent, the Vant Hoff factor, ‘i’, will be equal to one because the observed and normal molar masses will be same.
21. Inclusion of van’t Hoff factor, ‘F, modifies the equations for colligative properties as follows:
NCERT TEXTBOOK QUESTIONS SOLVED
2.1. Calculate the mass percentage of benzene (C6H6) and carbon tetrachloride (CCl4) if 22 g of benzene is dissolved in 122 g of carbon tetrachloride.
Ans: Mass of solution = Mass of C6H6 + Mass of CCl4
= 22 g+122 g= 144 g
Mass % of benzene = 22/144 x 100 =15.28 %
Mass % of CCl4 = 122/144 x 100 = 84.72 %
2.2. Calculate the mole fraction of benzene in solution containing 30% by mass in carbon tetrachloride.
Ans: 30% by mass of C6H6 in CCl4 => 30 g C6H6 in 100 g solution
.’. no. of moles of C6H6,(nC6h6) = 30/78 = 0.385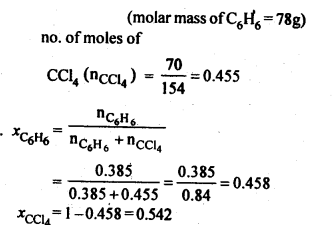
2.3. Calculate the molarity of each of the following solutions
(a) 30 g of Co(NO3)26H2O in 4·3 L of solution
(b) 30 mL of 0-5 M H2SO4 diluted to 500 mL.
Ans: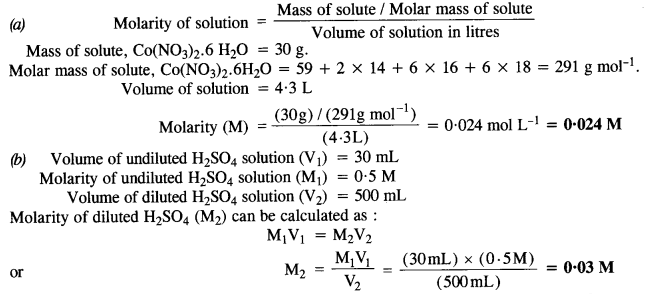
2.4. Calculate the mass of urea (NH2CONH2) required in making 2.5 kg of 0.25 molal aqueous solution.
Ans: 0.25 Molal aqueous solution to urea means that
moles of urea = 0.25 mole
mass of solvent (NH2CONH2) = 60 g mol-1
.’. 0.25 mole of urea = 0.25 x 60=15g
Mass of solution = 1000+15 = 1015g = 1.015 kg
1.015 kg of urea solution contains 15g of urea
.’. 2.5 kg of solution contains urea =15/1.015 x 2.5 = 37 g
2.5. Calculate
(a) molality
(b) molarity and
(c) mole fraction of KI if the density of 20% (mass/mass) aqueous KI solution is 1·202 g mL-1.
Ans:
Step I. Calculation of molality of solution
Weight of KI in 100 g of the solution = 20 g
Weight of water in the solution = 100 – 20 = 80 g = 0-08 kg
Molar mass of KI = 39 + 127 = 166 g mol-1.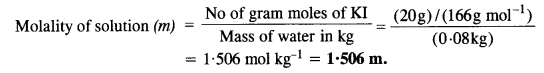
Step II. Calculation of molarity of solution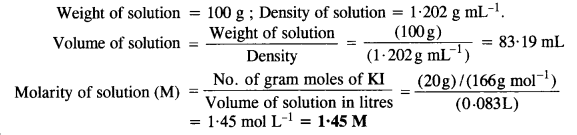
Step III. Calculation of mole fraction of Kl
2.6. H2 S, a toxic gas with rotten egg like smell, is used for the qualitative analysis. If the solubility of H2S in water at STP is 0.195 m, calculate Henry’s law constant.
Ans: Solubility of H2S gas = 0.195 m
= 0.195 mole in 1 kg of solvent
1 kg of solvent = 1000g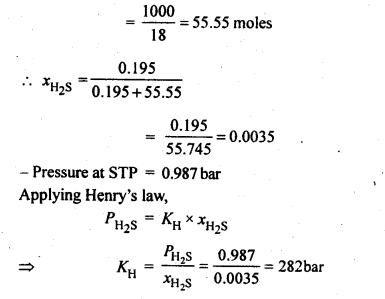
2.7. Henry’s law constant for CO2 in water is 1.67 x 108 Pa at 298 K. Calculate the quantity of CO2 in 500 mL of soda water when packed under 2.5 atm CO2 pressure at 298 K.
Ans.: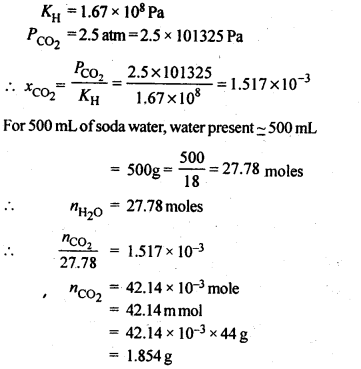
2.8 The vapour pressures of pure liquids A and B are 450 mm and 700 mm of Hg respectively at 350 K. Calculate the composition of the liquid mixture if total vapour pressure is 600 mm of Hg. Also find the composition in the vapour phase.
Ans:
Vapour pressure of pure liquid A (
Vapour pressure of pure liquid B (
Total vapour pressure of the solution (P) = 600 mm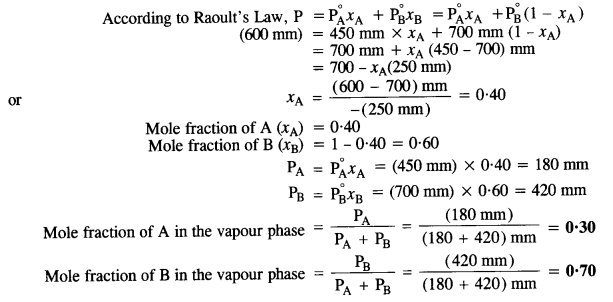
2.9. Vapour pressure of pure water at 298 K is 23.8 m m Hg. 50 g of urea (NH2CONH2) is dissolved in 850 g of water. Calculate the vapour pressure of water for this solution and its relative lowering.
Ans: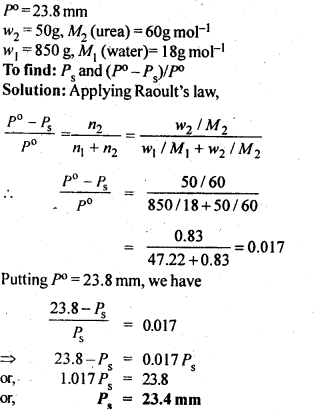
2.10. Boiling point of water at 750 mm Hg is 99.63°C. How much sucrose is to be added to 500 g of water such that it boils at 100°C.
Ans: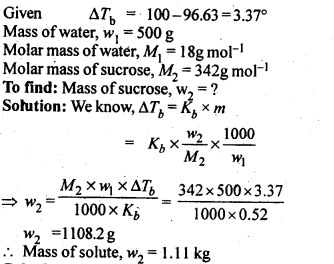
2.11 Calculate the mass of ascorbic acid (vitamin C, C6H8O6) to be dissolved in 75 g of acetic acid to lower its melting point by 1·5°C. (Kf for CH3COOH) = 3·9 K kg mol-1)
Ans: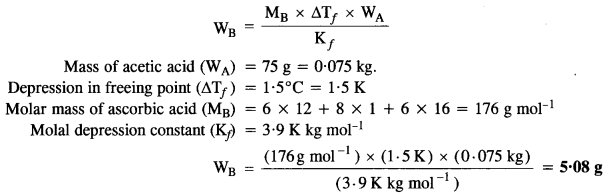
2.12. Calculate the osmotic pressure in pascals exerted by a solution prepared by dissolving 1.0 g of polymer of molar mass 185,000 in 450 mL of water at 37°C.
Ans: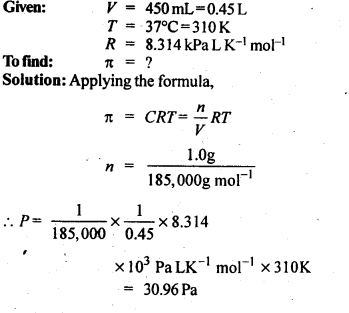
NCERT EXERCISES
2.1. Define the terra solution. How many types of solutions are formed? Write briefly about each type with an example.
Sol: A solution is a homogeneous mixture of two or more chemically non-reacting substances. Types of solutions: There are nine types of solutions.
Types of Solution Examples
Gaseous solutions
(a) Gas in gas Air, mixture of 02 and N2, etc.
(b) Liquid in gas Water vapour
(c) Solid in gas Camphor vapours in N2 gas, smoke etc.
Liquid solutions
(a) Gas in liquid C02 dissolved in water (aerated water), and 02 dissolved in water, etc.
(b) Liquid in liquid Ethanol dissolved in water, etc.
(c) Solid in liquid Sugar dissolved in water, saline water, etc.
Solid solutions
(a) Gas in solid Solution of hydrogen in palladium
(b) Liquid in solid Amalgams, e.g., Na-Hg
(c) Solid in solid Gold ornaments (Cu/Ag with Au)
2.2. Suppose a solid solution is formed between two substances, one whose particles are very large and the other whose particles are very small. What type of solid solution is this likely to be ?
Sol: The solution likely to be formed is interstitial solid solution.
2.3 Define the following terms:
(i) Mole fraction
(ii) Molality
(iii) Molarity
(iv) Mass percentage
Sol: (i) Mole fraction: It is defined as the ratio of the number of moles of the solute to the total number of moles in the solution. If A is the number of moles of solute dissolved in B moles of solvent, then Mole fraction of solute
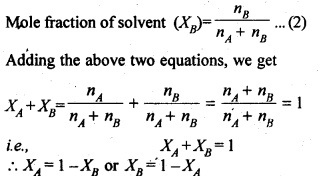
(ii) Molality: It is defined as die number of moles of a solute present in 1000g (1kg) of a solvent.![]()
NOTE: Molality is considered better way of expressing concentration of solutions, as compared to molarity because molality does not change with change in temperature since the mass of solvent does not vary with temperature,
(iii) Molarity: It is defined as the number of moles of solute present in one litre of solution.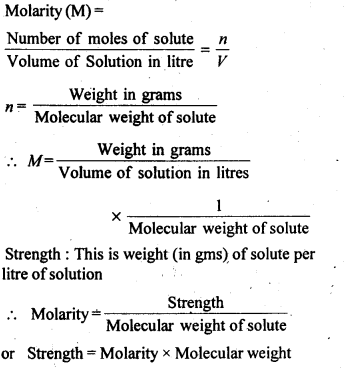
NOTE: Molarity is the most common way of expressing concentration of a solution in laboratory. However, it has one disadvantage. It changes with temperature because volume of a solution alters due to expansion and contraction of the liquid with temperature.
(iv) Mass percentage: It is the amount of solute in grams present in 100g of solution.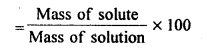
2.4. Concentrated nitric acid used in the laboratory work is 68% nitric acid by mass in aqueous solution. What should be the molarity of such a sample of acid if the density of the solution is 1·504 g mL-1 ?
Sol: Mass of HNO3 in solution = 68 g
Molar mass of HNO3 = 63 g mol-1
Mass of solution = 100 g
Density of solution = 1·504 g mL-1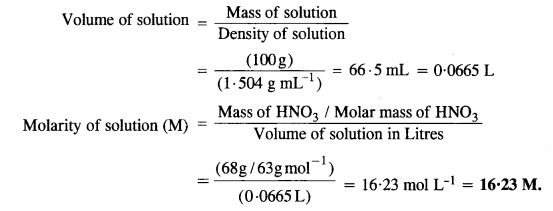
2.5. A solution of glucose in water is labelled as 10% w/w, what would be the molality and mole fraction of each component in the solution? If the density of solution is 1 .2 g m L-1, then what shall be the molarity of the solution?
Sol: 10 percent w/w solution of glucose in water means 10g glucose and 90g of water.
Molar mass of glucose = 180g mol-1 and molar mass of water = 18g mol-1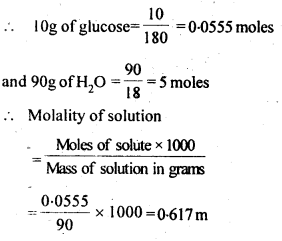

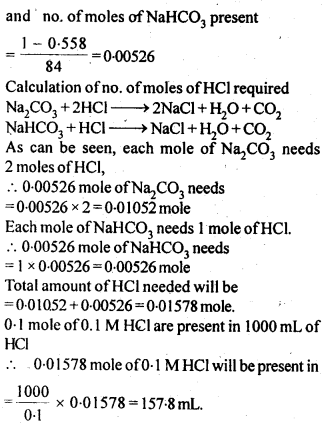
2.6. How many mL of 0.1 M HCl are required to react completely with 1 g mixture of Na2C03 and NaHCO3 containing equimolar amounts of both?
Sol: Calculation of no. of moles of components in the mixture.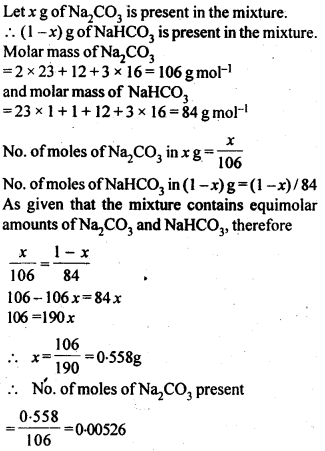
2.7. Calculate the percentage composition in terms of mass of a solution obtained by mixing 300 g of a 25% and 400 g of a 40% solution by mass.
Sol: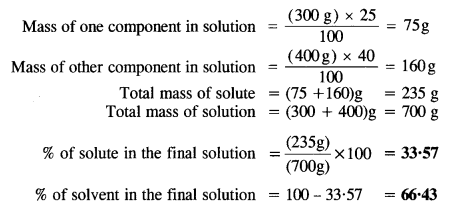
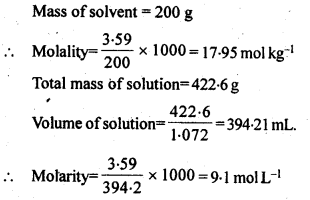
2.8. An antifreeze solution is prepared from 222.6 g of ethylene glycol, (C2 H6O2 ) and200 g of water. Calculate the molality of the solution. If the density of the solution is 1.072 g mL-1, then what shall be the molarity of the solution?
Sol: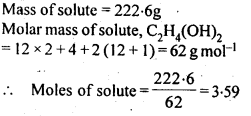
2.9. A sample of drinking water was found to be severely contaminated with chloroform (CHCl3), supposed to be a carcinogen. The level of contamination was 15 ppm (by mass).
(i) express this in percent by mass.
(ii) determine the molality of chloroform in the water sample.
Sol: 15 ppm means 15 parts in million (106) by mass in the solution.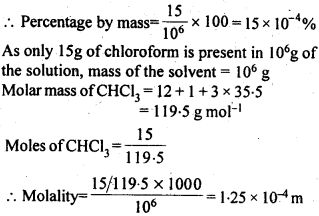
2.10. What role does the molecular interaction play in solution of alcohol in water?
Sol: In case of alcohol as well as water, the molecules are interlinked by intermolecular hydrogen bonding. However, the hydrogen bonding is also present in the molecules of alcohol and water in the solution but it is comparatively less than both alcohol and water. As a result, the magnitude of attractive forces tends to decrease and the solution shows positive deviation from Raoult’s Law. This will lead to increase in vapour pressure of the solution and also decrease in its boiling point.
2.11. Why do gases always tend to be less soluble in liquids as the temperature is raised?
Sol: When gases are dissolved in water, it is accompanied by a release of heat energy, i.e., process is exothermic. When the temperature is increased, according to Lechatlier’s Principle, the equilibrium shifts in backward direction, and thus gases becomes less soluble in liquids.
2.12. State Henry’s law and mention some of its important applications.
Sol:
Henry’s law: The solubility of a gas in a liquid at a particular temperature is directly proportional to the pressure of the gas in equilibrium with the liquid at that temperature.
or
The partial pressure of a gas in vapour phase is proportional to the mole fraction of the gas (x) in the solution. p = KHX
where KH is Henry’s law constant.
Applications of Henry’s law :
(i) In order to increase the solubility of CO2 gas in soft drinks and soda water, the bottles are normally sealed under high pressure. Increase in pressure increases the solubility of a gas in a solvent according to Henry’s Law. If the bottle is opened by removing the stopper or seal, the pressure on the surface of the gas will suddenly decrease. This will cause a decrease in the solubility of the gas in the liquid i.e. water. As a result, it will rush out of the bottle producing a hissing noise or with a fiz.
(ii) As pointed above, oxygen to be used by deep sea divers is generally diluted with helium inorder to reduce or minimise the painfril effects during decompression.
(iii) As the partial pressure of oxygen in air is high, in lungs it combines with haemoglobin to form oxyhaemoglobin. In tissues, the partial pressure of oxygen is comparatively low. Therefore, oxyhaemoglobin releases oxygen in order to carry out cellular activities.
2.13. The partial pressure of ethane over a solution containing 6.56 × 10-3 g of ethane is 1 bar. If the solution contains 5.00 × 10-2 g of ethane, then what shall be the partial pressure of the gas?
Sol: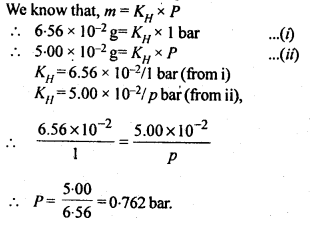
2.14. According to Raoult’s law, what is meant by positive and negative deviaitions and how is the sign of ∆solH related to positive and negative deviations from Raoult’s law?
Sol: Solutions having vapour pressures more than that expected from Raoult’s law are said to exhibit positive deviation. In these solutions solvent – solute interactions are weaker and ∆solH is positive because stronger A – A or B – B interactions are replaced by weaker A – B interactions. Breaking of the stronger interactions requires more energy & less energy is released on formation of weaker interactions. So overall ∆sol H is positive. Similarly ∆solV is positive i.e. the volume of solution is some what more than sum of volumes of solvent and solute.
So there is expansion in volume on solution formation.
Similarly in case of solutions exhibiting negative deviations, A – B interactions are stronger than A-A&B-B. So weaker interactions are replaced by stronger interactions so , there is release of energy i.e. ∆sol H is negative.
2.15. An aqueous solution of 2 percent non-volatile solute exerts a pressure of 1·004 bar at the boiling point of the solvent. What is the molecular mass of the solute ?
Sol:
According to Raoult’s Law,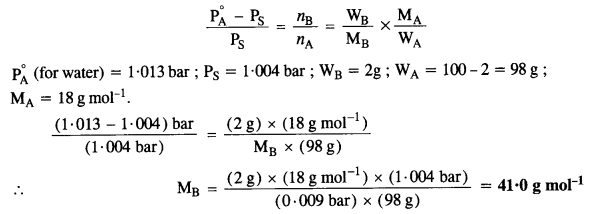
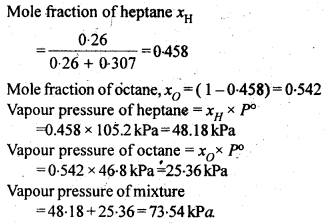
2.16 Heptane and octane form an ideal solution. At 373 K, the vapour pressures of the two liquid components are 105.2 kPa and 46.8 kPa respectively. What will be the vapour pressure of a mixture of 26.0 g of heptane and 35.0 g of octane?
Sol.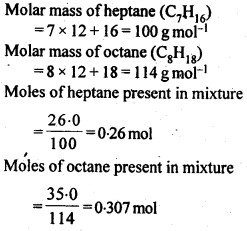
2.17. The vapour pressure of water is 12.3 kPa at 300 K. Calculate vapour pressure of 1 molal solution of a non-volatile solute in it
Sol: 1 molal solution of solute means 1 mole of solute in 1000g of the solvent.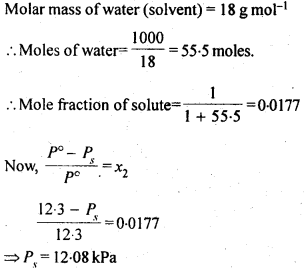
2.18. Calculate the mass of a non-volatile solute (molecular mass 40 g mol-1) that should be dissolved in 114 g of octane to reduce its pressure to 80%. (C.B.S.E. Outside Delhi 2008)
Sol: According to Raoult’s Law,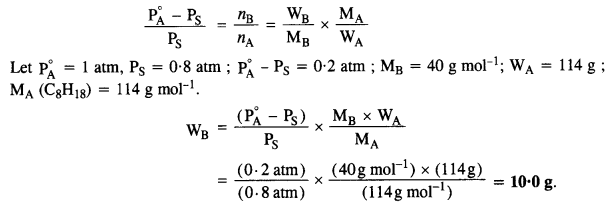
2.19. A solution containing 30g of non-volatile solute exactly in 90 g of water has a vapour pressure of 2.8 kPa at 298 K. Further, 18g of water is then added to the solution and the new of vapour pressure becomes 2.9 kPa at 298 K. Calculate
(i) molar mass of the solute.
(ii) vapour pressure of water at 298 K.
Sol: Let the molar mass of solute = Mg mol-1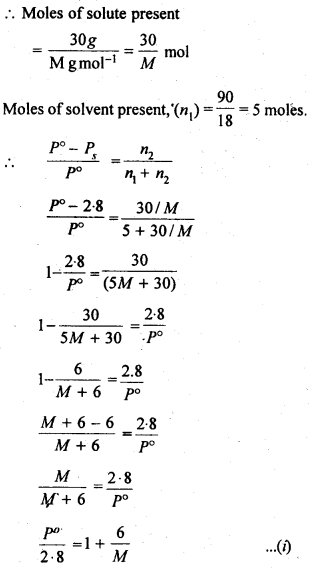
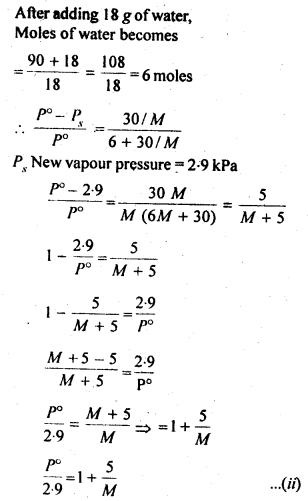
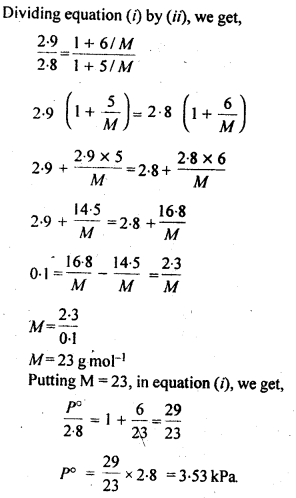
2.20. A 5% solution (by mass) of cane sugar in water has freezing point of 271 K. Calculate the freezing point of 5% glucose in water if freezing point of pure water is 273.15 K.
Sol: Mass of sugar in 5% (by mass) solution means 5gin 100g of solvent (water)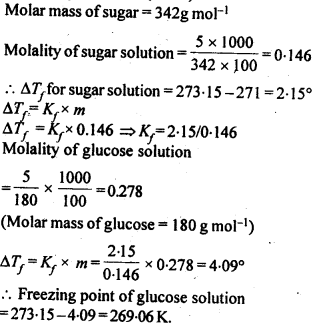
2.21. Two elements A and B form compounds having formula AB2 and AB4. When dissolved in 20g of benzene (C6H6), 1 g of AB2 lowers the freezing point by 2.3 K whereas 1.0 g of AB4 lowers it by 1.3 K. The molar depression constant for benzene is 5.1 K kg mol-1. Calculate atomic masses of A and B.
Sol: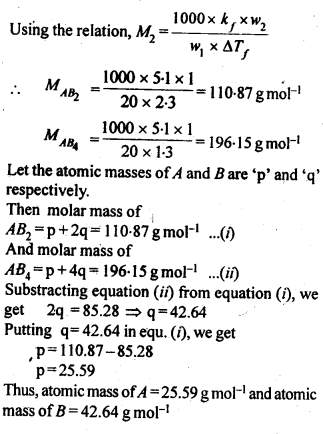
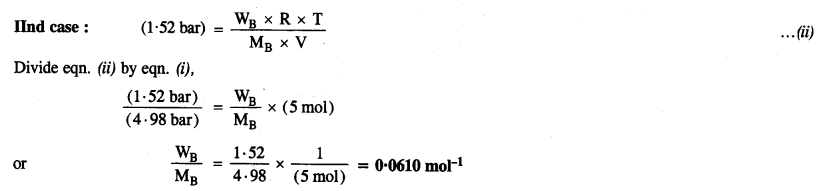
2.22. At 300 K, 36 g glucose present per litre in its solution has osmotic pressure of 4·98 bar. If the osmotic pressure of the solution is 1·52 bar at the same temperature, what would be its concentration?
Sol: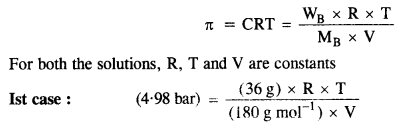
2.23. Suggest the most important type of intermolecular attractive interaction in the following pairs:
(i) n-hexane and n-octane
(ii) I2 and CCl4.
(iii) NaCl04 and water
(iv) methanol and acetone
(v) acetonitrile (CH3CN) and acetone (C3H60)
Sol: (i) Both w-hexane and n-octane are non-polar. Thus, the intermolecular interactions will be London dispersion forces.
(ii) Both I2 and CCl4 are non-polar. Thus, the intermolecular interactions will be London dispersion forces.
(iii) NaCl04 is an ionic compound and gives Na+ and Cl04– ions in the Solution. Water is a polar molecule. Thus, the intermolecular interactions will be ion-dipole interactions.
(iv) Both methanol and acetone are polar molecules. Thus, intermolecular interactions will be dipole-dipole interactions.
(v) Both CH3CN and C3H6O are polar molecules. Thus, intermolecular interactions will be dipole-dipole interactions.
2.24. Based on solute solvent interactions, arrange the following in order of increasing solubility in n-octane and explain. Cyclohexane, KCl, CH3OH, CH3CN.
Sol: n-octane (C8H18) is a non-polar liquid and solubility is governed by the principle that like dissolve like. Keeping this in view, the increasing order of solubility of different solutes is:
KCl < CH3OH < CH3C=N < C6H12 (cyclohexane).
2.25. Amongst the following compounds, identify which are insoluble, partially soluble and highly soluble in water?
(i) phenol
(ii) toluene
(iii) formic acid
(iv) ethylene glycol
(v) chloroform
(vi) pentanol
Sol: (i) Phenol (having polar – OH group) – Partially soluble.
(ii) Toluene (non-polar) – Insoluble.
(iii) Formic acid (form hydrogen bonds with water molecules) – Highly soluble.
(iv) Ethylene glycol (form hydrogen bonds with water molecules) Highly soluble.
(v) Chloroform (non-polar)- Insoluble.
(vi) Pentanol (having polar -OH) – Partially soluble.
2.26. If the density of lake water is 1·25 g mL-1, and it contains 92 g of Na+ ions per kg of water, calculate the molality of Na+ ions in the lake. (C.B.S.E. Outside Delhi 2008)
Sol: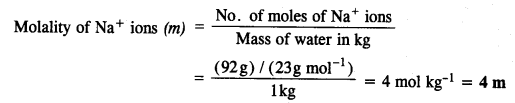
2.27. If the solubility product of CuS is 6 x 10-16, calculate the maximum molarity of CuS in aqueous solution.
Sol: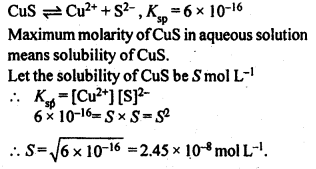
2.28. Calculate the mass percentage of aspirin (C9H8O4 in acetonitrile (CH3CN) when 6.5g of CHO is dissolved in 450 g of CH3CN.
Solution:
Mass of aspirin = 6.5 g
Mass of acetonitrile = 450 g
Then, total mass of the solution = (6.5 + 450) g = 456.5 g
Therefore, mass percentage of C9H8O4 =
= 1.424%
2.29. Nalorphene (C19H21NO3), similar to morphine, is used to combat withdrawal symptoms in narcotic users. Dose of nalorphene generally given is 1.5 mg. Calculate the mass of 1.5 x 10-3 m aqueous solution required for the above dose.
Solution: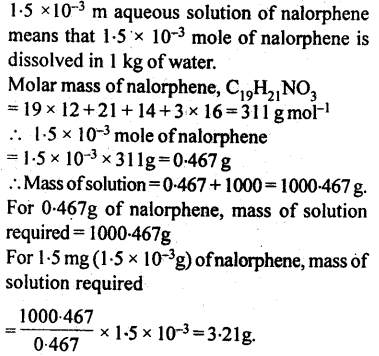
2.30. Calculate the amount of benzoic acid (C5H5COOH) required for preparing 250 mL of 0· 15 M solution in methanol.
Solution: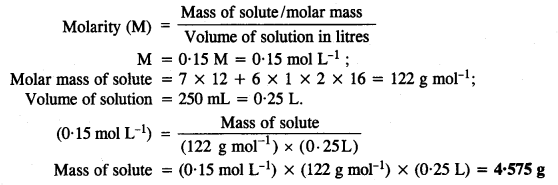
2.31. The depression in freezing point of water observed for the same amount of acetic acid, trichloroacetic acid and trifluoroacetic acid increases in the order given above. Explain briefly.
Solution: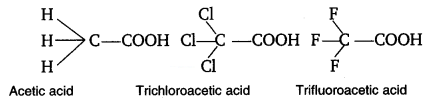
Fluorine being more electronegative than chlorine has the highest electron withdrawing inductive effect. Thus, triflouroacetic acid is the strongest trichloroacetic acid is second most and acetic acid is the weakest acid due to absence of any electron withdrawing group. Thus, F3CCOOH ionizes to the largest extent while CH3COOH ionizes to minimum extent in water. Greater the extent of ionization greater is the depression in freezing point. Hence, the order of depression in freezing point will be CH3COOH < Cl3CCOOH < F3CCOOH.
2.32. Calculate the depression in the freezing point of water when 10g of CH3CH2CHClCOOH is added to 250g of water. Ka = 1.4 x 1o-3 Kg = 1.86 K kg mol-1.
Solution:
Mass of solute (CH3CH2CHClCOOH) = 10 g
Molar mass of
CH3CH2CHClC00H = 4 × 12 + 7 × 1 + 1 × 35.5 + 2 × 16 = 48 + 7 + 35.5 + 32
= 122.5 g mol-1
\frac{\text { Mass / Molar mass }}{\text { Mass of solvent (Kg) }} =
= 0.326 m
Let α be the degree of dissociation of CH3CH2CHClCOOH then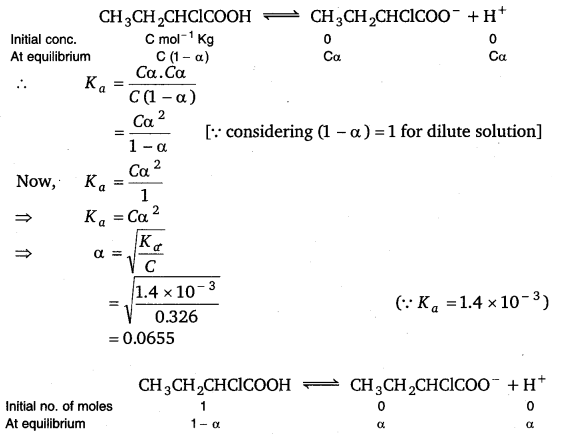
Total no. of moles after dissociation = 1 – α + α + α
= 1 + α
Van’t Hoff factor
Total no. of moles after dissociation
∴ i =
= 1 + α
= 1 + 0.0655
= 1.0655
Hence, the depression in the freezing point of water is given as:
ΔTf = i.Kfm
= 1.0655 × 1.86 kg mol-1 × 0.326 mol kg-1
= 0.65K
2.33. 19.5g of CH2FCOOH is dissolved in 500g of water. The depression in the freezing point of water observed is 1.0°C. Calculate the van’s Hoff factor and dissociation constant of fluoroacetic acid.
Solution:
Calculation of Van’t Hoff factor (i)
Given, w1 = 500 g = 0.5 kg, w2 = 19.5 g, Kf = 1.86 K kg mol-1, ΔTf = 1 K
Molar mass of CH2FCOOH (M2)
= 2 × 12 + 3 × 1 + 1 × 19 + 2 × 16
= 24 + 3 + 19 + 32
= 78 g mol-1
ΔTf = i Kf m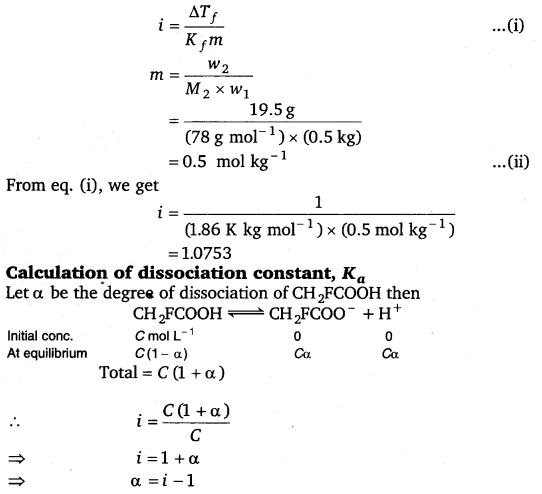
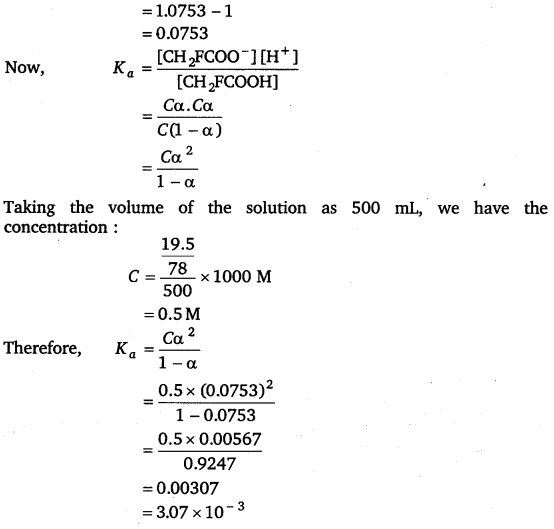
2.34. Vapour pressure of water at 293 K is 17·535 mm Hg. Calculate the vapour pressure of water at 293 K when 25 g of glucose is dissolved in 450 g of water.
Solution:
According to Raoult’s Law,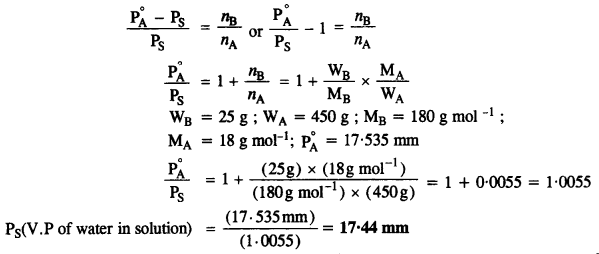
2.35. Henry’s law constant for the molality of methane in benzene at 298 K is 4.27 x 105 mm Hg. Calculate the solubility of methane in benzene at 298 K under 760 mm Hg.
Solution:
Here, p = 760 mm Hg, KH = 4.27 × 105 mm Hg (at 298 K)
According to Henry’s law, p = KHχ
χ =
=
= 177.99 × 10-5
= 178 × 10-5
Hence, the mole fraction of methane in benzene is 178 × 10-5.
2.36. 100g of liquid A (molar mass 140 g mol-1) was dissolved in 1000g of liquid B (molar mass 180g mol-1). The vapour pressure of pure liquid B was found to be 500 torr. Calculate the vapour pressure of pure liquid A and its vapour pressure in the solution if the total vapour pressure of the solution is 475 torr.
Solution:
Number of moles of liquid A, nA =
Number of moles of liquid B,nB =
Then, mole fraction of A, χA =
=
Mole fraction of B, χB = 1 – 0.114 = 0.886
Vapour pressure of pure liquid B,
Therefore, vapour pressure of liquid B in the solution,
PB =
= 500 × 0.886
= 443 torr
Total vapour pressure of the solution, ptotal = 475 torr
∴ Vapour pressure of liquid A in the solution,
PA = Ptotal – PB
= 475 – 443 = 32 torr
Now, PA =
⇒
= 280.7 torr
Hence, the vapour pressure of pure liquid A is 280.7 torr.
2.37. Vapour pressures of pure acetone and chloroform at 328 K are 741.8 mm Hg and 632.8 mm Hg respectively. Assuming that they form ideal solution over the entire range of composition, plot Ptotal, Pchlroform and Pacetone as a function of χacetone. The experimental data observed for different compositions of mixtures is: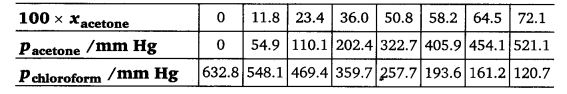
Plot this data also on the same graph paper. Indicate whether it has positive deviation or negative deviation from the ideal solution.
Solution: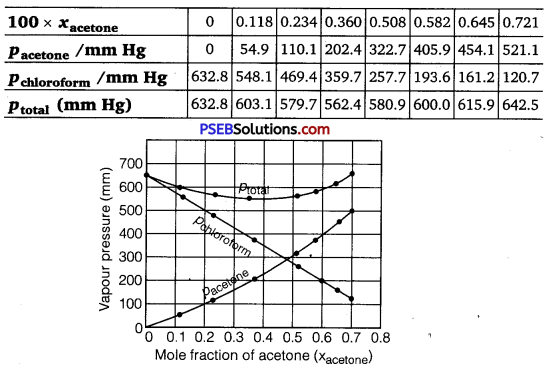
It can be observed from the graph that the plot for the ptotai of the solution curves downwards. Therefore, the solution shows negative deviation from the ideal behaviour.
2.38. Benzene and toluene form ideal solution over the entire range of composition. The vapour pressure of pure benzene and toluene at 300 K are 50.71 mm Hg and 32.06 mm Hg respectively. Calculate the mole fraction of benzene in vapour phase if 80g of benzene is mixed with 100g of toluene.
Solution:
Molar mass of benzene (C6H6) = 6 × 12 + 6 × 1 = 78g mol-1
Molar mass of toluene (C6H5CH3 ) = 7 × 12 + 8 × 1 = 92 g mol-1
No. of moles present in 80 g of benzene =
No. of moles present in 100 g of toluene =
Mole fraction of benzene, χC6H6, =
∴ Mole fraction of toluene,χC6H5CH35013 = 1 – 0.486 = 0.514
It is given that vapour pressure of pure benzene,
Vapour pressure of pure toluene,
Therefore, partial vapour pressure of benzene,
Ptotal = χC6H6 ×
= 0.486 × 50.71
= 24.645 mm Hg
Partial vapour pressure of toluene, PC6H5CH3 = χC6H5CH3 ×
= 0.514 × 32.06
= 16.479 mm Hg
Total vapour pressure of solution (p) = 24.645 + 16.479
= 41.124 mm Hg
Mole fraction of benzene in vapour phase
=
=
= 0.599 ≅ 0.6
2.39. The air is a mixture of a number of gases. The major components are oxygen and nitrogen with an approximate proportion of 20% is to 79% by volume at 298 K. The water is in equilibrium with air at a pressure of 10 atm. At 298 K if Henry’s law constants for oxygen and nitrogen are 3.30 x 107 mm and 6.51 x 107 mm respectively, calculate the composition of these gases in water.
Solution:
Percentage of oxygen (O2) in air = 20%
Percentage of nitrogen (N2) in air = 79%
Also, it is given that water is in equilibrium with air at a total pressure of 10 atm that is, (10 × 760) mm = 7600 mm
Therefore, partial pressure of oxygen,
PO2 =
= 1520 mm Hg
Partial pressure of nitrogen, pN2 =
= 6004 mm Hg
Now, according to Henry’s law,
p = KH.χ
For oxygen: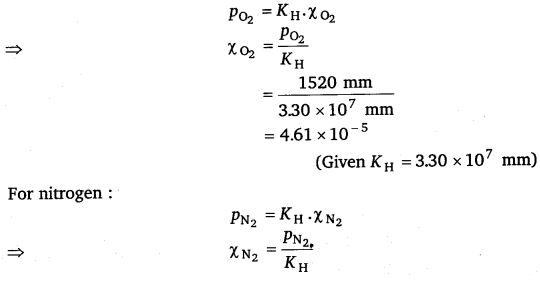
(Given KH = 6.51 × 107 mm)
= 9.22 × 10-5
Hence, the mole fractions of oxygen and nitrogen in water are 4.61 × 10-5 and 9.22 × 10-5 respectively.
2.40. Determine the amount of CaCl2 (i = 2.47) dissolved in 2.5 litre of water such that its osmotic pressure is 0.75 atm at 27°C.
Solution:
We know that,
π = i
⇒ π = i
⇒ w =
Given,
π = 0.75 atm
V = 2.5L
i = 2.47
T = (27 + 273)K = 300K
R = 0.0821 L atm K-1mol–
Molar mass of CaCl2(M) = 1 × 40 + 2 × 35.5 = 111 g mol -1
Therefore, w =
Hence the required amount of CaCl2 is3.42g
2.41. Determine the osmotic pressure of a solution prepared by dissolving 25 mg of K2SO4 in 2 litre of water at 25°C, assuming that it is completely dissociated. (C.B.S.E. 2013)
Solution:
Step I. Calculation of Van’t Hoff factor (i)
K2SO4 dissociates in water as :