Phenols – Nomenclature, Preparation and Properties
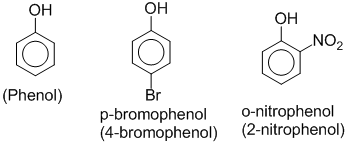
Phenols are hydroxyl derivatives of benzene in which –OH group is directly attached to benzene ring. Eg.
If –OH group is attached to the side chain of an aromatic ring then it isn’t considered as phenol. This type of compounds are known as aromatic alcohol. Eg.

Contents [hide]
- Nomenclature of phenols
- General methods of preparation of phenol
- Physical properties of phenol
- Chemical properties of phenol
- Reactions due to phenolic(-OH) group
- Reactions due to benzene ring
- Special reactions of phenol
- References
Nomenclature of phenols

General methods of preparation of phenol
1. From diazonium salt (Laboratory method):
Phenol is prepared in the laboratory by warming aq. solution of benzene diazonium chloride.

2. From chlorobenzene (Dow’s process):
Chlorobenzene when heated with aq. solution of NaOH (or Na2CO3) at about 3500C under 300 atmospheric pressure gives sodium phenoxide which upon acidification gives phenol.

3. From sodium salicylate:
Sodium salicylate on distillation with sodalime undergoes decarboxylation to form sod. phenoxide which on acidification gives phenol.

4. From Grignard reagent:
When oxygen is bubbled through an ethereal solution of phenyl magnesium bromide, it forms an addition product which upon acidic hydrolysis gives phenol.

5. From methoxy benzene(anisole):
Methoxy benzene when heated with HI gives phenol.

Q) How will you convert phenol into anisole (methoxy benzene) and vice-versa.

Physical properties of phenol
1. Solubility of phenols:
Phenols are slightly (sparingly) soluble in water though it can form intermolecular H-bond with water molecules. Limited solubility is mainly due to hydrophobic nature of benzene ring.

2. Melting and boiling point of phenols:
The melting and boiling points of phenols are higher than the arenes and holoarenes of comparable molecular masses. This is due to the presence of intermolecular H-bond between phenol molecules.

Chemical properties of phenol
In general, chemical reactions of phenols are of two types.
1. Reactions due to phenolic(-OH) group.
2. Reactions due to benzene ring.
Reactions due to phenolic(-OH) group
1. Acidic nature of phenols:
Phenols turn blue litmus red i.e. phenols behave as weak acids and react with metals and alkalies as follows:

Phenols do not react with Na2CO3 or NaHCO3 as they are weaker acids than carboxylic acids.
Q) Phenols are stronger acids than alcohols, why?
Phenols are more acidic alcohols. This can be explained by considering the relative stabilities of phenols and phenoxide ions as compared to alcohols and alkoxide ions respectively.
Phenols as well as phenoxide ions both are resonance stabilized and their resonating structures are given below:

The resonating structures of phenol involves charge separation, whereas in case of phenoxide ion there is no charge separation. From this it is clear that phenoxide ion is more stabilized by resonance than phenol. Therefore, in the dissociation of phenol, equilibrium shifts to the forward direction and forms relatively high concentration of H+ ions and behave as strong acids compared to alcohols.

On the other hand, in the case of alcohols neither alcohols nor alkoxide ions are stabilized by resonance, and hence they behave as weaker acids than phenol.

Hence,

2. Reaction with zinc dust:
When phenol is heated with zinc dust, benzene is formed.

3. Reaction with ammonia: In the presence of anhydrous ZnCl2, phenol reacts with ammonia at high temperature to give aniline.

4.Reaction with acid chlorides or acid anhydrides: (Acylation)
Phenols react with acid chloride in presence of pyridine or with acid anhydrides in the presence of mineral acids like H2SO4 or base pyridine to form esters.

5. Reaction with benzoyl chloride: (Benzoylation)
Phenols react with benzoyl chloride in the presence of aqueous NaOH to form phenyl benzoate.

6. Reaction with phosphorus pentachloride:
Phenols react with PCl5 to produce chlorobenzene.

Reactions due to benzene ring
Electrophilic substitution reaction of phenol:
The resonating structures of phenol are:

It is clear from the above resonating structures that ortho and para positions are relatively rich in electron density and hence incoming electrophile attacks at these positions. Thus, -OH group is an ortho/para directing group towards electrophilic substitution reaction.
1. Halogenation: Phenols react with halogens to form poly halogen substituted compounds. For example, phenol gives white ppt. of 2,4,6-tribromophenol when treated with bromine water.

2. Nitration: Phenol when nitrated with concentrated nitric acid and conc. H2SO4 gives 2,4,6-trinitrophenol (picric acid).

3. Sulphonation: Sulphonation of phenol takes place mainly at ortho-position at low temperature and at para-position at high temperature.

4. Friedel-Craft’s alkylation: Phenols react with alkyl halides in the presence of anhy.AlCl3 to form mainly para-product with a small amount of the ortho-product.

Special reactions of phenol
1. Kolbe’s reaction (Carboxylation reaction):
Sodium phenoxide when heated with CO2 at1350C under a pressure of 4-7 atm, sodium salicylate is obtained which when acidified gives salicylic acid.

Salicylic acid is the starting material for the manufacture of 2-acetoxybenzoic acid (aspirin), the well known analgesic. Aspirin is obtained from salicylic acid by reacting it with acetic anhydride.

2. Reimer-Tiemann reaction: When phenol is refluxed with chloroform and aq. NaOH at 600C, a mixture of o-hydroxybenzaldehyde and p-hydroxybenzaldehyde is obtained. This reaction is called Reimer-Tiemann reaction. 
3. Reaction with phthalic anhydride:
Phenol on condensation with phthalic anhydride in the presence of conc. H2SO4 gives phenolphthalein.

4. Coupling reaction:
Phenols react with benzene diazonium chloride in slightly alkaline medium and at low temperature to form coloured compounds called azo dyes. This reaction is called coupling reaction.

Note: Arene diazonium salts react with highly reactive aromatic compounds like phenol, aniline, etc. to form highly colored azo-compound, (Ar-N=N-Ar). This reaction is known as coupling reaction.
5. Catalytic hydrogenation: Phenol gets hydrogenated in the presence of nickel catalyst at 1500C to give cyclohexanol.

6. Oxidation: On exposure to air, phenol gets slowly oxidized and turns pink in colour.

7. Reaction with ferric chloride: Phenols react with ferric chloride solution to form water soluble coloured complexes.

8. Condensation(polymerization) of phenol with formaldehyde (methanal):
Phenol condenses with formaldehyde in the presence of an acid or basic catalyst to form a polymer called Bakelite.
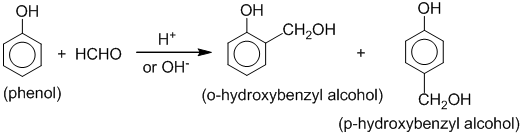
Formation of linear polymer:

Formation of cross-linked polymers:

References
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Morrison, R.T. , Boyd, R.N., Organic Chemistry, Sixth edition, Prentice-Hall of India Pvt. Ltd., 2008.
- Sthapit, M.K., Pradhananga, R.R., Foundations of Chemistry, Vol 1 and 2, Fourth edition, Taleju Prakashan, 2005.
- https://www.vedantu.com/chemistry/preparation-of-phenol#:~:text=In%20this%20method%2C%20benzene%20sulfonic,aqueous%20acid%20to%20make%20phenol.
- https://www.cliffsnotes.com/study-guides/chemistry/organic-chemistry-ii/phenols-and-aryl-halides/synthesis-of-phenols
