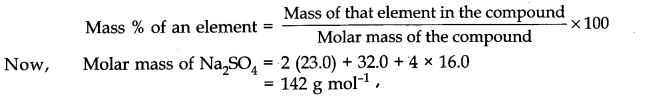Hydrocarbons : Alkanes, Alkenes and Alkynes – Preparation and Properties
Hydrocarbons are the organic compounds containing carbon and hydrogen only. Hydrocarbons are broadly classified as – aliphatic, alicyclic and aromatic hydrocarbons. In this note, we are going to study about preparation and properties of aliphalic hydrocarbons i.e open chain hydrocarbons. Aliphatic hydrocarbons are further classified as – saturated hydrocarbons (alkanes) and unsaturated hydrocarbons (alkenes and alkynes).
Contents [hide]
Alkanes (saturated hydrocarbons)Preparation of alkanesChemical properties of alkanes
AlkenesPreparation of alkenes
Chemical properties of alkenes
AlkynesPreparation of alkynes
Chemical properties of alkynes
Test of unsaturation ( i.e Test of alkenes and alkynes)
Kolbe’s electrolysis method for the preparation of Alkanes, alkenes and Alkynes
References
Alkanes (saturated hydrocarbons)
Preparation of alkanes
1. From haloalkanes
ether, an alkane containing double number of carbon atoms than in haloalkane is formed.
This reaction is called Wurtz reaction.

Q) Identify A.

b. By reduction: Alkyl halides when reduced with Zn/HCl , H2/Ni, etc. give alkanes. Eg.

2. By catalytic hydrogenation of alkenes and alkynes:

3. By decarboxylation of (salt of) carboxylic acid:
is eliminated from the molecule to give alkane. This reaction is called decarboxylation reaction. Eg.
Chemical properties of alkanes
For example, chlorine reacts with methane in presence of sunlight or heat to form four different halogen
derivatives.

2. Nitration: Alkane reacts with nitric acid at high temperature to form nitroalkane. Eg.

3. Sulphonation : When alkane is heated with fuming sulphuric acid, alkane sulphonic acid is formed. Eg.

large amount of heat.

Eg.

Lower alkanes when heated with limited supply of air at 350-5000C form aldehydes. Eg.

Alkenes
Preparation of alkenes
1. By dehydrohalogenation of alkyl halide ( elimination reaction):
halogen atom is eliminated from adjacent carbon atoms to give alkene. Eg.

This rule states that when there is a chance of formation of more than one alkene, then the more substituted
alkene is formed as major product.
Eg. In the dehydrohalogenation of 2-bromobutane, but-2-ene is the major product over but-1-ene.

2. By dehydration of alcohols:
alkene when it is heated with dehydrating agent like sulphuric acid(H2SO4), phosphoric acid(H3PO4),
alumina(Al2O3) etc. eg.

eg.

3. By controlled hydrogenation of alkynes:
formed. Eg.

Note: Lindlar’s catalyst = Pd+BaSO4+quinoline
Chemical properties of alkenes
1. Addition of hydrogen (Catalytic hydrogenation):
formed. This reaction is called catalytic hydrogenation.

2. Addition of halogens:
Eg. ethene reacts with Br2 in presence of CCl4 to give 1,2-dibromoethane. In this reaction reddish brown
colour of bromine is discharged. Hence this is a test reaction of ethene (alkene).

3. Addition of hydrogen halides ( halogen acids)(HCl, HBr, HI):
Alkene reacts with halogen acids to give alkyl halide (haloalkane). Eg.

When alkene is unsymmetrical then the addition takes place according to Markovnikov’s rule.
Markovnikov’s rule:
of the reagent goes to that double bonded carbon which has lesser number of hydrogen atoms.
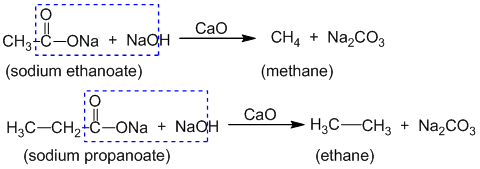
For example: The addition of HBr to propene gives 2- bromopropane instead of 1- bromopropane.
Other example:

Peroxide effect or anti-Markovnikov’s rule:
bonded carbon atom having more number of hydrogen. This phenomenon of anti- Markovnikov’s addition
of HBr caused by the presence of peroxide is known as peroxide effect or anti- Markovnikov’s rule.

4. Addition of water [Catalytic hydration]:
Alkenes react with water in presence of dilute mineral acid as catalyst to form alcohol. Eg.

5. Addition of sulphuric acid:
Alkenes react with concentrated sulphuric acid to give alkyl hydrogen sulphate. Eg.

6. Ozonolysis:
molecules of carbonyl compounds (aldehyde or ketone). This process of formation of ozonide and it’s
decomposition to give carbonyl compounds is called ozonolysis.

7. Polymerization:
undergoing polymerization are called monomers. The polymers are high molecular weight large molecules
made by the polymerization of monomers.
Ethene polymerizes to form polyethene.

Alkynes
Preparation of alkynes
1. By direct combination of elements (i.e. carbon and hydrogen):
atmosphere of hydrogen gas.

2. By dehydrohalogenation of vicinal dihalides:
When vicinal dihalides are treated with alcoholic KOH, alkynes are formed by dehydrohalogenation. Eg.

Note: Vicinal dihalide– Compounds that contain two hydrogen atoms on adjacent carbon atoms.
3. By heating trihalogen derivatives with silver powder:
Trihaloalkanes like chloroform and iodoform when heated with silver powder form alkynes. Eg.

Chemical properties of alkynes
1. Addition of hydrogen ( Reduction):
When alkyne is heated with hydrogen in presence of Ni, Pt or Pd catalyst, alkane is formed. Eg.

poisoned by sulphur to give alkene. Eg.

2. Addition of halogen acids(HX):
Alkynes react with two molecules of halogen acids according to Markovnikov’s rule to give dihaloalkane. Eg.

3. Addition of water : Catalytic hydration:
rearranges to give aldehyde or ketone.

For example, ethyne gives ethanal (i.e. aldehyde).

Propyne gives propanone (i.e. ketone).

4. Reaction with bromine solution:
discharged. This is test reaction of alkyne (unsaturated compound).

5. Polymerization reaction:
compounds.
Eg. Three molecules of ethyne (acetylene) polymerize to give benzene.

6. Formation of acetylides (Acidic nature of acetylene):
Acetylene is acidic in nature because it releases H+ easily.
a. Action with sodium metal:
Acetylene reacts with molten Na metal to form sodium acetylide.

b. Action with ammonical silver nitrate solution (Tollen’s reagent):
which have white ppt.

c. Action with ammonical cuprous chloride solution:
Acetylene reacts with ammonical cuprous chloride solution to form copper acetylide which have red ppt.


Test of unsaturation ( i.e Test of alkenes and alkynes)
1. Bromine decolorization test:
unsaturated compounds (alkenes or alkynes). Therefore, this reaction is used to detect the presence of
multiple bond in a molecule. Eg.


2. Baeyer’s test ( Oxidation with alkaline solution of KMnO4):
Alkene reacts with Baeyer’s reagent to form glycol, where pink colour of the potassium permanganate is
discharged. Therefore this reaction is used as test reaction of alkenes.

Similarly, alkynes also discharge the pink colour of Baeyer’s reagent.
* Baeyer’s reagent oxidizes ethyne to oxalic acid and the pink colour of KMnO4 is discharged.

* Other alkynes react with Baeyer’s reagent to give two molecules of carboxylic acids. Eg.

Q) Give a suitable test to distinguish following pairs.
a. ethyne and ethane
b. ethene and ethyne.
Kolbe’s electrolysis method for the preparation of Alkanes, alkenes and Alkynes
Alkanes, alkenes and alkynes are prepared by electrolysis of salt of monocarboxylic acid, dicarboxylic acid
and unsaturated dicarboxylic acid respectively.
1. Preparation of alkanes:
solution. Eg.
Ethane is produced at anode during the electrolysis of an aqueous solution of sodium or potassium acetate
as follows:

2. Preparation of alkenes:
solution. Eg.
Ethene is produced at anode during the electrolysis of an aqueous solution of sodium or potassium
succinate as follows:

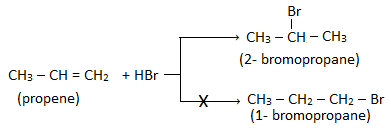
3. Preparation of alkynes:
solution. Eg.
Ethyne is produced at anode during the electrolysis of an aqueous solution of sodium or potassium maleate (i.e. salt
of maleic acid) as follows:
References
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Sharma, M.L., Chaudary, P.N., A Text Book of B.Sc. Chemistry, Second edition (Volume one and two), Ekta Books, Kathmandu, 2004.
- Sthapit, M.K., Pradhananga, R.R., Foundations of Chemistry, Vol 1 and 2, Fourth edition, Taleju Prakashan, 2005.
- https://en.wikipedia.org/wiki/Acetylene
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Map%3A_Organic_Chemistry_(Smith)/Chapter_01%3A_Structure_and_Bonding/1.9%3A_Ethane%2C_Ethylene%2C_and_Acetylene
- https://www.britannica.com/science/hydrocarbon/Nomenclature-of-alkenes-and-alkynes.
- Kolkatta, 2007.
- https://socratic.org/organic-chemistry-1/ways-to-draw-and-represent-molecules/condensed-structure
- https://www.britannica.com/science/homologous-series