Coordination Compounds
1. Coordination compounds contain a central atom (or cation) which is coordinated to a suitable number of anions or neutral molecules and usually retain their identity in solution as well as in solid state. These may be a positively charged, negatively charged or a neutral species,
[Co(NH3)6]3+, [NiCl4]2-, [Ni(CO)4] etc.
2. In 1893, Werner proposed a theory to explain the structure and bonding in coordination compounds:
(a) In coordination compounds, metals show two types of valencies : Primary valency and secondary valency.
(b) Primary valencies are ionisable.
(c) Secondary valencies are not ionisable.
(d) This theory was successful to very limited extent and could not explain many aspects of coordination compounds.
3. In modern formulations, such spatial , arrangements are called coordination polyhedra.
The species within the square bracket are coordination entities or complexes and the ions outside the square brackets are called counter ions.
4. The compounds which have the same molecular formula but differ in their structural arrangements are known as isomers.
5. The types of isomerism shown by coordination compounds are :
(a) Geometrical (or cis-trans) isomerism: Two coordination compounds are said to be geometrical isomers, when they differ in the arrangement of their ligands in space. When two identical ligands occupy adjacent position, the isomer is called ‘cis-form’ and when they arranged opposite to one another, the isomer is called ‘trans-form’.
(b) Optical isomerism shown by the chiral molecule, i.e., the molecules which do not have plane of symmetry e.g., [Cr(ox)3]3-.
(c) Linkage isomerism occurs in complexes when an ambidentate ligand is present in the coordination sphere, e.g., [CO(NH3)5N02]2+ and [Co(NH3)5(-ONO)]2+.
(d) Coordination isomerism occurs in those complexes which are made of cationic and anionic coordination entities due to the interchange of ligands between the cation and anion entities, e.g., [CO(NH3)6] [Cr(CN)6] and [Co(CN)6] [Cr(NH3)6].
(e) Ionisation isomerism, is due to the exchange of ions in coordination sphere of metal ion and the ions outside the coordination sphere. These two isomers give different ions in aqueous solution, e.g,
[Co(NH3)5Br]2+ S042- and [Co(NH3)5(S04)]+ Br–
(f) Solvate or hydrate isomerism occurs when water is a part of coordination entity or is outside it, e.g., CrCl3-6H20 has three isomers.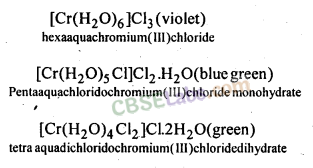
6. Magnetic properties:
(i) Inner orbital (low spin) complexes are those complexes in which hybrid orbitals of metal are formed by hybridisation of (n-1) d, ns and np-orbitals, e.g., [Fe(CN)6]4-, [CO(NH3)6]3+, [Cr(NH3)6]3+, [Fe(CN)6]2+, [Fe(H20)6]2+, [(MnCCN)6]3-, etc.
(ii) Outer orbital (high spin) complexes are those comp lexes in which the hybrid orbitals of metal are formed by hybridisation ns, np and nd-vacant orbitals, e.g., [MnF6]3-, [FeF6]3-, [Ni(NH3)6]2+,[Ni(H20)6]2+, etc.
7. Assumptions of Crystal Field Theory:
(a) The ligands are assumed to be point charges.
(b) The interaction between the point charges and the electrons of the central metal are electrostatic in nature.
(c) The 5d-orbitals in an isolated gaseous metal ion have the same energy, i.e., they are degenerate.
8. The stability of a coordination compound [MLn] is measured in terms, of its stability constant
For overall reaction,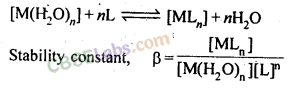
9. Drawbacks of Crystal Field Splitting:
(a) From the assumption that ligands are point charges, it follows that anionic ligands should exert greatest splitting effect. But anionic ligands actually are found at the low end of the Spectro-chemical series.
(b) It does not take into account for the covalent character of bonding between the ligand and the central atom.
10. Bonding in metal carbonyls: In metal carbonyl, the metal carbon (M – C) bond possesses both the σ- and π-bond character.
11. Importance and applications of coordination compounds:
(a) In many quantitative and qualitative chemical analysis.
(b) In extraction processes of metals, like silver and gold.
(c) Purification of metals like Ni can be achieved through formation and subsequent decomposition of their coordination compounds.
(d) In biological systems the pigment responsible for photosynthesis is chlorophyll, is a coordination compound of magnesium. Hemoglobin, coordination compound, of Fe, acts as a oxygen carrier.
(e) Case of chelate therapy in medicinal chemistry.
What is Valance Bond (VB) Theory?
According to the valence bond theory,
Electrons in a molecule occupy atomic orbitals rather than molecular orbitals. The overlapping of atomic orbitals results in the formation of a chemical bond and the electrons are localized in the bond region due to overlapping.
History of Valence Bond Theory
The Lewis approach to chemical bonding failed to shed light on the formation of chemical bonds. Also, valence shell electron pair repulsion theory (or VSEPR theory) had limited applications (and also failed in predicting the geometry corresponding to complex molecules).
In order to address these issues, the valence bond theory was put forth by the German physicists Walter Heinrich Heitler and Fritz Wolfgang London. The Schrodinger wave equation was also used to explain the formation of a covalent bond between two hydrogen atoms. The chemical bonding of two hydrogen atoms as per the valence bond theory is illustrated below.

This theory focuses on the concepts of electronic configuration, atomic orbitals (and their overlapping), and the hybridization of these atomic orbitals. Chemical bonds are formed from the overlapping of atomic orbitals wherein the electrons are localized in the corresponding bond region.
The valence bond theory also goes on to explain the electronic structure of the molecules formed by this overlapping of atomic orbitals. It also emphasizes that the nucleus of one atom in a molecule is attracted to the electrons of the other atoms.
Postulates of Valence Bond Theory
The important postulates of the valence bond theory are listed below.
- Covalent bonds are formed when two valence orbitals (half-filled) belonging to two different atoms overlap on each other. The electron density in the area between the two bonding atoms increases as a result of this overlapping, thereby increasing the stability of the resulting molecule.
- The presence of many unpaired electrons in the valence shell of an atom enables it to form multiple bonds with other atoms. The paired electrons present in the valence shell do not take participate in the formation of chemical bonds as per the valence bond theory.
- Covalent chemical bonds are directional and are also parallel to the region corresponding to the atomic orbitals that are overlapping.
- Sigma bonds and pi bonds differ in the pattern that the atomic orbitals overlap in, i.e. pi bonds are formed from sidewise overlapping whereas the overlapping along the axis containing the nuclei of the two atoms leads to the formation of sigma bonds.
The formation of sigma and pi bonds is illustrated below.

It can be noted that sigma bonds involve the head-to-head overlapping of atomic orbitals whereas pi bonds involve parallel overlapping.
Number of Orbitals and Types of Hybridization
According to VBT theory the metal atom or ion under the influence of ligands can use its (n-1)d, ns, np, or ns, np, nd orbitals for hybridization to yield a set of equivalent orbitals of definite geometry such as octahedral, tetrahedral, square planar and so on. These hybrid orbitals are allowed to overlap with ligand orbitals that can donate electron pairs for bonding.
| Coordination Number | Type of Hybridisation | Distribution of Hybrid Orbitals in Space |
| 4 | sp3 | Tetrahedral |
| 4 | dsp2 | Square planar |
| 5 | sp3d | Trigonal bipyramidal |
| 6 | sp3d2 | Octahedral |
| 6 | d2sp3 | Octahedral |
Applications of Valence Bond Theory
- The maximum overlap condition which is described by the valence bond theory can explain the formation of covalent bonds in several molecules.
- This is one of its most important applications. For example, the difference in the length and strength of the chemical bonds in H2 and F2 molecules can be explained by the difference in the overlapping orbitals in these molecules.
- The covalent bond in an HF molecule is formed from the overlap of the 1s orbital of the hydrogen atom and a 2p orbital belonging to the fluorine atom, which is explained by the valence bond theory.
Limitations of Valence Bond Theory
The shortcomings of the valence bond theory include
- Failure to explain the tetravalency exhibited by carbon.
- No insight is offered on the energies of the electrons.
- The theory assumes that electrons are localized in specific areas.
- It does not give a quantitative interpretation of the thermodynamic or kinetic stabilities of coordination compounds.
- No distinction between weak and strong ligands.
- No explanation for the colour exhibited by coordination compounds
What is the valence bond theory?
It is a theory which describes chemical bonding. VBT states that the overlap of incompletely filled atomic orbitals leads to the formation of a chemical bond between two atoms. The unpaired electrons are shared and a hybrid orbital is formed.
What are the shortcomings of VBT?
The valence bond theory fails to explain the tetravalency of carbon and also fails to provide insight into the energies corresponding to the electrons. The theory also assumes that the electrons are localized in certain areas.
What are the merits of the valence bond theory?
The condition of maximum overlap described by the VBT can be used to explain how covalent bonds are formed in many molecules. The theory can also offer insight into the ionic character of chemical bonds.
How are sigma and pi bonds formed?
Sigma bonds are formed from the head-to-head overlapping of the atomic orbitals participating in the bond. Pi bonds, on the other hand, involve a parallel overlapping of the atomic orbitals.
What are the assumptions of the valence bond theory?
Can valence bond theory determine shape?
Who discovered the valence bond theory?
What is the difference between VSEPR and valence bond theory?
Does valence bond theory predict the bond angle?
Does valence bond theory explain the resonance in a compound?
What is Crystal Field Theory?
Crystal field theory describes
the net change in crystal energy resulting from the orientation of d orbitals of a transition metal cation inside a coordinating group of anions also called ligands.
A major feature of transition metals is their tendency to form complexes. A complex may be considered as consisting of a central metal atom or ion surrounded by a number of ligands. The interaction between these ligands with the central metal atom or ion is subject to crystal field theory.
Crystal field theory was established in 1929 and treats the interaction of metal ion and ligand as a purely electrostatic phenomenon where the ligands are considered as point charges in the vicinity of the atomic orbitals of the central atom. Development and extension of crystal field theory taken into account the partly covalent nature of bonds between the ligand and metal atom mainly through the application of molecular orbital theory. Crystal field theory is often termed ligand field theory.
Table of Content
- Overview of Crystal Field Theory
- High Spin and Low Spin
- Recommended Videos
- Crystal Field Splitting in Octahedral Complex
- Crystal Field Splitting in Tetrahedral Complex
- Crystal Field Stabilization Energy
- Crystal Field Stabilization Energy Table
- Frequently Asked Questions – FAQs
Overview of Crystal Field Theory
In order to understand clearly the crystal field interactions in transition metal complexes, it is necessary to have knowledge of the geometrical or spatial disposition of d orbitals. The d-orbitals are fivefold degenerate in a free gaseous metal ion. If a spherically symmetric field of negative ligand filed charge is imposed on a central metal ion, the d-orbitals will remain degenerate but followed by some changes in the energy of the free ion.
A summary of the interactions is given below.
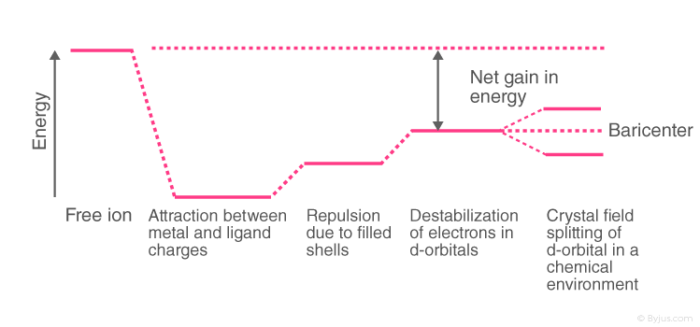
Crystal Field Splitting
Crystal field theory was proposed which described the metal-ligand bond as an ionic bond arising purely from the electrostatic interactions between the metal ions and ligands. Crystal field theory considers anions as point charges and neutral molecules as dipoles.
When transition metals are not bonded to any ligand, their d orbitals degenerate that is they have the same energy. When they start bonding with other ligands, due to different symmetries of the d orbitals and the inductive effect of the ligands on the electrons, the d orbitals split apart and become non-degenerate.
High Spin and Low Spin
The complexion with the greater number of unpaired electrons is known as the high spin complex, the low spin complex contains the lesser number of unpaired electrons. High spin complexes are expected with weak field ligands whereas the crystal field splitting energy is small Δ. The opposite applies to the low spin complexes in which strong field ligands cause maximum pairing of electrons in the set of three t2 atomic orbitals due to large Δo.
- High spin – Maximum number of unpaired electrons.
- Low spin – Minimum number of unpaired electrons.
Example: [Co(CN)6]3- & [CoF6]3-
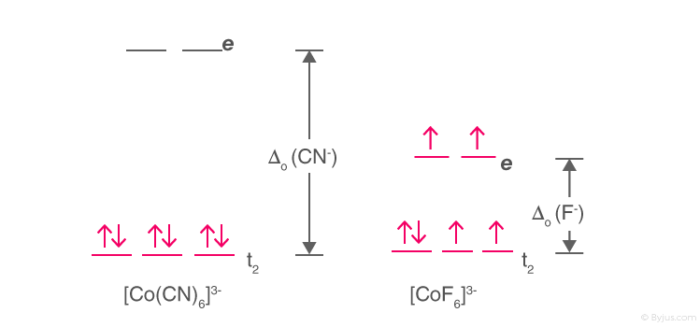
High Spin and Low Spin Complex
- [Co(CN)6]3- – Low spin complex
- [CoF6]3- – High spin complex
The pattern of the splitting of d orbitals depends on the nature of the crystal field. The splitting in various crystal fields is discussed below:
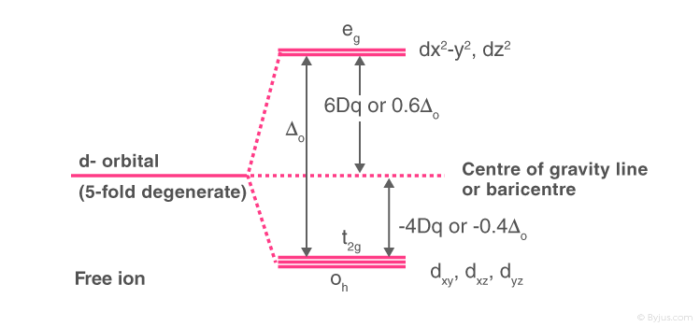
Crystal Field Splitting in Octahedral Complex
- In the case of an octahedral coordination compound having six ligands surrounding the metal atom/ion, we observe repulsion between the electrons in d orbitals and ligand electrons.
- This repulsion is experienced more in the case of dx2-y2 and dz2 orbitals as they point towards the axes along the direction of the ligand.
- Hence, they have higher energy than average energy in the spherical crystal field.
- On the other hand, dxy, dyz, and dxz orbitals experience lower repulsions as they are directed between the axes.
- Hence, these three orbitals have less energy than the average energy in the spherical crystal field.
Thus, the repulsions in octahedral coordination compound yield two energy levels:
- t2g– set of three orbitals (dxy, dyz and dxz) with lower energy
- eg – set of two orbitals (dx2-y2 and dz2) with higher energy
Crystal Field Splitting in Octahedral Complex
This splitting of degenerate level in the presence of ligand is known as crystal field splitting. The difference between the energy of t2g and eg level is denoted by “Δo” (subscript o stands for octahedral). Some ligands tend to produce strong fields thereby causing large crystal field splitting whereas some ligands tend to produce weak fields thereby causing small crystal field splitting.
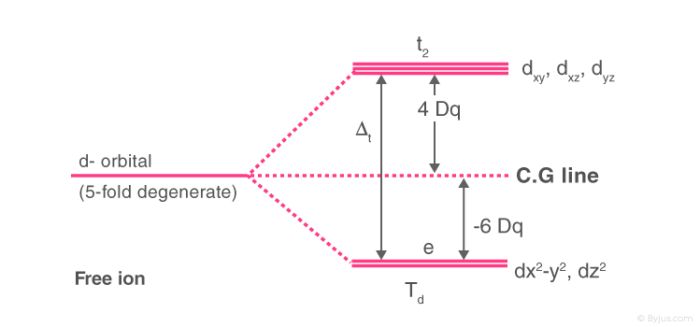
Crystal Field Splitting in Tetrahedral Complex
The splitting of fivefold degenerate d orbitals of the metal ion into two levels in a tetrahedral crystal field is the representation of two sets of orbitals as Td. The electrons in dx2-y2 and dz2 orbitals are less repelled by the ligands than the electrons present in dxy, dyz, and dxz orbitals. As a result, the energy of dxy, dyz, and dxz orbital sets are raised while that of the dx2-y2 and dz2 orbitals are lowered.
- There are only four ligands in Td complexes and therefore the total negative charge of four ligands and hence the ligand field is less than that of six ligands.
- The direction of the orbitals does not coincide with the directions of the ligands approach to the metal ion.
Crystal Field Splitting in Tetrahedral Complex
Thus, the repulsions in tetrahedral coordination compound yield two energy levels:
- t2– set of three orbitals (dxy, dyz and dxz) with higher energy
- e – set of two orbitals (dx2-y2 and dz2) with lower energy
The crystal field splitting in a tetrahedral complex is intrinsically smaller in an octahedral filed because there are only two thirds as many ligands and they have a less direct effect of the d orbitals. The relative stabilizing effect of e set will be -6Dq and the destabilizing effect of t2 set will be +4Dq
Crystal Field Stabilization Energy
In a chemical environment, the energy levels generally split as directed by the symmetry of the local field surrounding the metal ion. The energy difference between the eg and t2g levels is given as or 10Dq. It states that each electron that goes into the lower t2g level stabilizes the system by an amount of -4Dq and the electron that goes into eg level destabilizes the system by +6Dq. That is the t2g is lowered by 4Dq and the eg level is raised by +6Dq.
For example, the net change in energy for d5 and d10 systems will be zero as shown below.
d5 :- 3(-4Dq) + 2(+6Dq) = -12Dq + 12Dq = 0
d10 :- 6(-4Dq) + 4(+6Dq) = -24Dq + 24Dq = 0
The decrease in energy caused by the splitting of the energy levels is called the “Ligand Field Stabilization Energy (LFSE)”.
Crystal Field Stabilization Energy Table
The crystal field stabilization energies for some octahedral and tetrahedral complexes of 3d metal ions are tabulated below.
| Electronic Configuration | Octahedral Complex | Tetrahedral Complex | ||
| Weak Field (-Dq) | Strong Field (-Dq) | Weak Field (-Dq) | Strong Field (-Dq) | |
| d0 | 0 | 0 | 0 | 0 |
| d1 | 4 | 4 | 6 | 6 |
| d2 | 8 | 8 | 12 | 12 |
| d3 | 12 | 12 | 8 | (18)* |
| d4 | 6 | 16 | 4 | (24)* |
| d5 | 0 | 20 | 0 | (20)* |
| d6 | 4 | 24 | 6 | (16)* |
| d7 | 8 | 18 | 12 | 12 |
| d8 | 12 | 12 | 8 | 8 |
| d9 | 6 | 6 | 4 | 4 |
| d10 | 0 | 0 | 0 | 0 |
Thus, the crystal field splitting depends on the field produced by the ligand and the charge on the metal ion. An experimentally determined series based on the absorption of light by coordination compound with different ligands known as spectrochemical series has been proposed. Spectrochemical series arranges ligands in order of their field strength as:
I– < Br– < Cl– < SCN– < F– < OH– < C2O42- < H2O < NCS– < EDTA4- < NH3 < en < CN–< CO
Filling of d-orbitals takes place in the following manner; the first three electrons are arranged in t2g level as per the Hund’s rule. The fourth electron can either enter into the t2g level giving a configuration of t2g4eg0 or can enter the eg orbital giving a configuration of t2g3eg1. This depends on two parameters magnitude of crystal field splitting, Δo and pairing energy, P. The possibilities of the two cases can better be explained as
- Δo > P – Electron enters in the t2g level giving a configuration of t2g4eg0. Ligands producing this configuration are known as strong field ligands and form low spin complexes.
- Δo < P – Electron enters in the eg level giving a configuration of t2g3eg1. Ligands producing this configuration are known as weak field ligands and form high spin complexes.
Frequently Asked Questions – FAQs
Who discovered crystal field theory?
Hans Bethe, a physicist, developed the crystal field theory (CFT) for crystalline solids in 1929.
What does crystal field theory explain?
Crystal field theory (CFT) describes the breaking of degeneracies of electron orbital states, typically d or f orbitals, caused by a static electric field generated by a surrounding charge distribution (anion neighbours).
What are the limitations of Crystal Field Theory?
Some limitations of CFT are as follows:
- This theory only considers the d-orbitals of a central atom. The s and p orbits are not taken into account in this study.
- The theory fails to explain the behaviour of certain metals, which exhibit large splitting while others exhibit minor splitting. For example, the theory provides no explanation for why H2O is a stronger ligand than OH–.
- The theory excludes the possibility of p bonding. This is a significant disadvantage because it is found in many complexes.
- The orbits of the ligands have no significance in the theory. As a result, it cannot explain any properties of ligand orbitals or their interactions with metal orbitals.
What are the advantages of Crystal Field Theory?
The following are some of the advantages of crystal field theory.
- This theory can be used to describe the stability of complexes. The greater the crystal field splitting energy, the greater the stability.
- Complexes’ colour and spectra can be explained using this theory.
- This theory explains complexes’ magnetic properties.
Why is CFT superior to VBT?
The mixing of orbitals during bond formation was explained by Valence Bond Theory (VBT). The explanation was primarily based on hybridisation concepts. While Crystal Field Theory explains how orbitals split as ligands approach metal ions, VBT was unable to adequately explain magnetic behaviour. It was unable to explain the formation of outer orbital and inner orbital complexes. CFT, on the other hand, explained everything
No comments:
Post a Comment